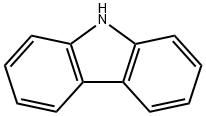
Carbazole
- CAS No.
- 86-74-8
- Chemical Name:
- Carbazole
- Synonyms
- DIBENZOPYRROLE;9-AZAFLUORENE;Diphenylenimide;DIPHENYLENIMINE;Skf 20091;CARBAZOLE;usafek-600;USAF ek-600;'LGC' (2409);Diphenylimid
- CBNumber:
- CB4854492
- Molecular Formula:
- C12H9N
- Molecular Weight:
- 167.21
- MDL Number:
- MFCD00004960
- MOL File:
- 86-74-8.mol
- MSDS File:
- SDS
| Melting point | 243-246 °C (lit.) |
|---|---|
| Boiling point | 355 °C (lit.) |
| Density | 1.1 |
| vapor pressure | 400 mm Hg ( 323 °C) |
| refractive index | 1.6192 (estimate) |
| Flash point | 220 °C |
| storage temp. | Store below +30°C. |
| solubility | acetone: soluble50mg/mL |
| form | Crystalline Powder, Flakes, or Chunks |
| pka | 17.00±0.30(Predicted) |
| color | Beige-yellow or beige-brownish |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Merck | 14,1790 |
| BRN | 3956 |
| Dielectric constant | 1.3(Ambient) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, nitrogen oxides, potassium hydroxide. |
| InChIKey | UJOBWOGCFQCDNV-UHFFFAOYSA-N |
| LogP | 3.84 at 22℃ and pH7 |
| CAS DataBase Reference | 86-74-8(CAS DataBase Reference) |
| EWG's Food Scores | 3-6 |
| FDA UNII | 0P2197HHHN |
| Proposition 65 List | Carbazole |
| IARC | 2B (Vol. 32, Sup 7, 71, 103) 2013 |
| NIST Chemistry Reference | Carbazole(86-74-8) |
| EPA Substance Registry System | Carbazole (86-74-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H341-H351-H413 | |||||||||
| Precautionary statements | P201-P202-P273-P280-P308+P313-P405 | |||||||||
| Hazard Codes | Xn,N,T | |||||||||
| Risk Statements | 22-36/37/38-40-50/53-63-43-23/24/25-45-67-68-51/53 | |||||||||
| Safety Statements | 26-36-60-61-36/37-24/25-23-53-45-36/37/39 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | FE3150000 | |||||||||
| F | 10 | |||||||||
| Hazard Note | Harmful | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29339990 | |||||||||
| Toxicity | LD50 orally in rats: >5 g/kg (Eagle, Carlson) | |||||||||
| NFPA 704 |
|
Carbazole price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.20255 | Carbazole for synthesis | 86-74-8 | 5G | $33.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.20255 | Carbazole for synthesis | 86-74-8 | 250g | $82.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46100 | Carbazole VETRANAL?, analytical standard | 86-74-8 | 250mg | $63.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1096702 | Carprofen Related Compound A United States Pharmacopeia (USP) Reference Standard | 86-74-8 | 50mg | $1380 | 2024-03-01 | Buy |
| TCI Chemical | C0032 | Carbazole >97.0%(GC) | 86-74-8 | 25g | $28 | 2024-03-01 | Buy |
Carbazole Chemical Properties,Uses,Production
Introduction
Carbazole and its derivatives are a class of important nitrogen-containing heterocyclic compounds possessing various unique properties and biological activity.
Carbazole is a colorless small scale crystal, insoluble in water and inorganic acid, slightly soluble in ethanol, ether, acetone, benzene (in high temperature), soluble in chloroform, glacial acetic acid, carbon disulfide, pyridine and furfural, etc. It has intense fluorescence and prolonged phosphorescence under ultraviolet light.
Extraction
Sulfuric acid method
Dissolve crude anthracene in chlorobenzene or other solvents to remove soluble phenanthrenes, quinones, etc. Insoluble anthracene and carbazoles are then reacted with concentrated sulfuric acid, and then carbazole sulfate is generated departing from the anthracene. The carbazole sulfate is hydrolyzed, filtered and dried to obtain carbazole.
Solvent-rectification method
Dissolve crude anthracene with heavy benzene, and remove soluble phenanthrene, quinone and other substances. Insoluble anthracene and carbazole are rectified in a rectification tower to obtain a mixture containing 85 to 90% of carbazole with a yield of 65%.
Pyridine solvent method
Dissolve crude anthracene with heavy benzene, and remove soluble phenanthrene, quinone and other substances. Then pyridine is used as a solvent to filter off insoluble anthracene at 90°C, and the filtrate is then crystallized to obtain crude carbazole. The crude carbazole can be treated with chlorobenzene or other solvents to obtain carbazole with yield of 97 to 99%.
Production
a. Graebe-Ullmann:
Diazotization from amino diphenylamine, and nitrogen gas heated off to generate carbazole. Reported by Graebe and Ullmann in 1896.
b. Bucherer:
Co-thermal reaction of aryl hydrazine and naphthol in the presence of sodium bisulfite. Reported by Bucherer in 1904.
c. Borsche Drechsel:
Hydrazone is synthesized by condensation of Benzoquinone and cyclohexanone, and the latter cyclized to tetrahydrocarbazole under acidic conditions and then catalyzed by dehydrogenation to generate carbazole. Reported by Drechsel and Borsche in 1868 and 1904 respectively.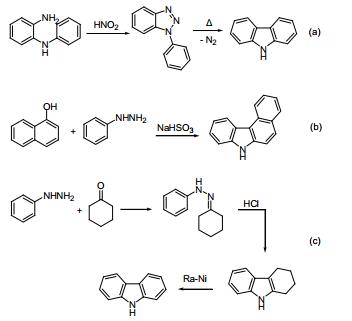
Thanks to the development of the catalytic dehydrogenation method, the Borsche method has become a carbazole synthetic route with simple operation, mild conditions, low cost and high yield, and has a high industrial production value.
Because of its unique structure and physicochemical properties, carbazole has aroused great interest among researchers. In recent years, novel monoand poly-substituted carbazole derivatives have been found to have good anti-tumor and anti-convulsant activities, showing broad application prospects. The demand for carbazole and its derivatives has been increasing. In the last decade of the 20th century, a large number of novel precious metals and transition metal catalysts have been used in the synthesis of carbazoles and their derivatives, which greatly enriched the synthetic methods and lay the foundation of synthesizing more complex carbazole derivatives. Nowadays, not only carbazole, but tens of thousands of carbazole derivatives are synthesized by new methods, and are widely used in the pharmaceutical and dye industries.
Chemical Properties
white crystals or light brown powder
Chemical Properties
In the laboratory method, the ring-closure dehydrogenation of o-aminobiphenyl is made from ammonia or chlorobenzene. In the industrial production method, 90% of the world's carbazole is obtained from coal tar; it can also be synthesized from o-aminobiphenyl, and then purified by recrystallization from xylene. (1) Synthesis method: 1-phenyl-1,2,3-benzotriazole is prepared by using o-aminodiphenylamine as raw material and treated with nitrous acid. After heating, nitrogen is lost to form carbazole. (2) Sulfuric acid method: Dissolve the crude anthracene with chlorobenzene or other solvents. The phenanthrene, fluorene and other substances in the crude anthracene are separated from anthracene and carbazole due to insolubility. Anthracene and carbazole are added to sulfuric acid for reaction. Then it forms carbazole sulfate with sulfuric acid and separates from anthracene. After hydrolyzing carbazole sulfate, the finished product is obtained by filtration and drying. (3) Solvent-rectification method: The crude anthracene is dissolved in the heavy benzene (160~200℃) by-product of coking, and the phenanthrene, fluorene and other substances in the crude anthracene are separated from anthracene and carbazole. High-temperature rectification is carried out in the distillation tower, and after one rectification, a product containing 85% to 90% of carbazole can be obtained, and the yield is 65%.
Uses
Intermediate in production of dyes and UVsensitive photographic plates.
Uses
Carbazole and its derivatives are widely used as an intermediate in synthesis of pharmaceuticals, agrochemicals, dyes, pigments and other organic compounds.?carbazole is also used in luminescence chemistry as a photosensitizing and additional charge transport material. Carbazole structure is a motif in pharmaceuticals such as carvedilol used to treat high blood pressure and to prevent cardiac arrhythmias and angina.
Uses
Important dye intermediate. Used in making photographic plates sensitive to ultraviolet light. Reagent for lignin, carbohydrates, and formaldehyde.
Definition
carbazole: A white crystalline compoundfound with anthracene,C12H9N; m.p. 238°C; b.p. 335°C. It isused in the manufacture of dyestuffs.
Application
Carbazole can be used to produce dyes, pigments, photoconductors, photosensitive materials, special inks, etc. The pigment produced with it is permanent purple RL, which is widely used in the coloring of automobile topcoat and high temperature resistant plastics, and has the advantages of high temperature resistance and ultraviolet light resistance. The dyestuffs sulphur vat blue RNX and Haichang blue produced with it have excellent fastness indicators, especially the fastness to chlorine bleaching. The blue varieties include carbazole IDM, carbazole LR, carbazole LB, and carbazole L3B. , the black varieties have carbazole black D. It also produces carbazole bisoxazine violet, a blue-violet pigment used in coatings, printing inks, carbon paper, and more. Carbazole is used in the production of sulfide reduced blue RNX, direct lightfast blue FFRL, FFGL, etc. It can also make leather, N-vinylcarbazole plastics, pesticides and insecticides tetranitrocarbazole, chlorinated carbazole, and UV-sensitive photographic dry films. In addition, carbazole has been increasingly used in the development of emerging optoelectronic new materials. The use of carbazole can prepare organic nonlinear optics (NLO) materials, organic electroluminescence (OEL) materials, photorefractive materials, containing Bifunctional system of carbazole chromophore, carbazole-containing photorefractive small molecular glass, etc.
Definition
A white crystalline compound used in the manufacture of dyestuffs.
Synthesis Reference(s)
Tetrahedron Letters, 14, p. 761, 1973 DOI: 10.1016/S0040-4039(01)95705-3
General Description
White crystals, plates, leaflets or light tan powder. Sublimes readily. Exhibits strong fluorescence and long phosphorescence on exposure to ultraviolet light.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Carbazole is an extremely weak base. Carbazole is incompatible with strong oxidizing agents. Carbazole reacts with nitrogen oxides. Potassium hydroxide fusion yields a salt.
Hazard
Possible carcinogen.
Fire Hazard
Flash point data for Carbazole are not available; however, Carbazole is probably combustible.
Safety Profile
intraperitoneal route. A flammable liquid.
Solubility in organics
Very weak alkaline. Slightly soluble in ethanol, ether and benzene, soluble in quinoline, pyridine and acetone, slightly soluble in cold benzene, glacial acetic acid, chloroform, carbon disulfide and gasoline, soluble in concentrated sulfuric acid without decomposition, slightly soluble in petroleum ether, chlorinated hydrocarbons and acetic acid.
Purification Methods
Dissolve carbazole (60g) in conc H2SO4 (300mL), extract with three 200mL portions of *benzene, then stir this into 1600mL of an ice-water mixture. The precipitate is filtered off, washed with a little water, dried, recrystallised from *benzene and then from pyridine/*benzene [Feldman et al. J Am Chem Soc 73 4341 1951]. It has also been recrystallised from EtOH or toluene, sublimed in vacuum, zone-refined, and purified by TLC. [UV: Armarego in Physical Methods in Heterocyclic Chemistry (Ed Katritzky, Academic Press) Vol III 158 1971, Beilstein 20/8 V 9.]
Carbazole Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Allgreen Chemical Co.,LTD | +86-37155567971 +86-13633837469 | info@allgreenchem.com | China | 5986 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Speranza Chemical Co., Ltd. | +86-86-075521030354 +8618688942810 | sophieliu@speranzachem.com | China | 723 | 55 |
| AB PharmaTech,LLC | 323-480-4688 | United States | 989 | 55 | |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 559 | 58 |
Related articles
- The physicochemical properties of carbazole
- Carbazole, also known as 9-nitrogen (hetero) fluorene (dibenzopyrrole), has a molecular formula of C12H9N and a molecular weig....
- Apr 13,2022
- Synthesis of Carbazole
- Carbazole is a tricyclic heterocycle with a 14π electron ring system, comprised of two benzene rings fused at 2,3 and 4,5 site....
- Jan 24,2022
View Lastest Price from Carbazole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Carbazole
86-74-8
|
US $0.00 / Kg/Bag | 1ASSAYS | 99% | 300mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2023-07-25 | Carbazole
86-74-8
|
US $20.00-10.00 / KG | 1KG | 93%/95%/98% | 20TONS | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-03-22 | Carbazole
86-74-8
|
US $20.00 / kg | 1kg | 99% | 5000 Ton | Hebei Duling International Trade Co. LTD |
86-74-8(Carbazole)Related Search:
1of4