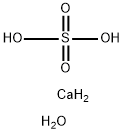
Calcium sulfate dihydrate
- CAS No.
- 10101-41-4
- Chemical Name:
- Calcium sulfate dihydrate
- Synonyms
- GYPSUM;CALCIUM SULPHATE DIHYDRATE;selenite;CSD;gypse;annaline;satinite;alabaster;lightspar;c.i. 77231
- CBNumber:
- CB5110067
- Molecular Formula:
- CaH6O5S
- Molecular Weight:
- 158.18
- MDL Number:
- MFCD00149625
- MOL File:
- 10101-41-4.mol
- MSDS File:
- SDS
| Melting point | 128°C -1.5H₂O |
|---|---|
| Boiling point | 163°C -2H₂O |
| Density | 2.32 |
| Flash point | 163°C -2H₂O |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.01 M at 20 °C, clear, colorless |
| form | powder |
| color | White |
| Specific Gravity | 2.32 |
| Odor | at 100.00?%. odorless |
| PH | 7.0 (50g/l, H2O, 20℃)(slurry) |
| Water Solubility | 2 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,1706 |
| Solubility Product Constant (Ksp) | pKsp: 4.5 |
| Exposure limits | ACGIH: TWA 10 mg/m3 |
| Dielectric constant | 2.5(Ambient) |
| Stability | Stable. Incompatible with aluminium, strong acids. |
| InChIKey | PASHVRUKOFIRIK-UHFFFAOYSA-L |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 10101-41-4(CAS DataBase Reference) |
| FDA UNII | 4846Q921YM |
| NIST Chemistry Reference | calcium sulfate(10101-41-4) |
| EPA Substance Registry System | Calcium sulfate dihydrate (10101-41-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P305+P351+P338-P321 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/21/22-36/37/38 | |||||||||
| Safety Statements | 22-24/25-36-26 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | EW4150000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28332990 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 10000 mg/kg | |||||||||
| NFPA 704 |
|
Calcium sulfate dihydrate price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 12056 | Calcium sulfate dihydrate puriss., meets analytical specification of NF, E 516, 99.0-101.0% (based on anhydrous substance) | 10101-41-4 | 1kg | $116 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02161 | Calcium sulfate dihydrate precipitated for analysis EMSURE? | 10101-41-4 | 500g | $124 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02160 | Calcium sulfate dihydrate precipitated EMPROVE? ESSENTIAL Ph Eur,BP,E 516 | 10101-41-4 | 1kg | $132 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02161 | Calcium sulfate dihydrate precipitated for analysis EMSURE? | 10101-41-4 | 25kg | $2490 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.02160 | Calcium sulfate dihydrate precipitated EMPROVE? ESSENTIAL Ph Eur,BP,E 516 | 10101-41-4 | 25kg | $1493 | 2024-03-01 | Buy |
Calcium sulfate dihydrate Chemical Properties,Uses,Production
Outline
Calcium sulfate dehydrate is also known as "natural anhydrite". Chemical formula is CaSO4. The molecular weight is 136.14. It is rhombic system crystal. The relative density is 2.960, refractive index is 1.569,1.575,1.613. Another is soluble anhydrite: melting point is 1450℃, the relative density is 2.89, refractive index is 1.505,1.548, When be incandescent, it can decompose. Its hemihydrate is commonly known as "plaster of Paris", "plaster", it is white amorphous powder, relative density is 2.75. Its dehydrate is commonly known as "gypsum", as a white crystal or powder, relative density is 2.32, refractive index is 1.521,1.523,1.530, when be heated to 163℃, it can lose all crystal water. It is slightly soluble in water, hydrochloric acid, nitric acid, it is soluble in hot sulfuric acid, it is insoluble in alcohol. Natural product is soluble in alkaline sulphates, sodium thiosulfate, aqueous solution of ammonium salt. Method: natural anhydrite is derived by the reaction of CaO with SO3 under red-hot. Soluble anhydrous gypsum is derived by CaSO4·2H2O which heated at 200℃ to constant. Hemihydrate gypsum is derived by the calcination dehydration. Dihydrate is reacted by calcium chloride and ammonium sulfate. The main purpose of calcium sulphate: natural anhydrite is used for medicine; soluble anhydrite can be used for interior decoration, it can also be used to prepare chemicals, beverages, etc; hemihydrate is used for construction materials, but also can be used in plaster statues, ceramics material; its dihydrate is used to product hemihydrate, fillers, etc.
The above information is edited by the chemicalbook of Wang Xiaodong.
Agricultural application
Mineral gypsum (CaSO4·2H20) is the most commonly used amendment for sodic and alkali soil reclamation. Gypsum reclaims alkali soils and is thus beneficial to agriculture. Much of the high-grade gypsum comes from the wet process phosphoric acid industry. Given the high demand for phosphorus in the world, many industrial plants have been set up in many countries. This has given rise to huge amounts of phosphogypsum waste, giving rise to disposal problems. Mineral gypsum also poses the same problem.
Gypsum is a hydrated form of calcium sulphate (CaSO4·2H20). It is a white or yellowish solid which can be ground to particle sizes from 10 to 100 mesh. Pure gypsum has 18.6% sulphur and 23.2% calcium. A 70 to 80% pure commercial agricultural grade gypsum contains 13 to 15% sulphur, 16 to 19% calcium and varying amounts of impurities such as the oxides of iron, aluminum, calcium and magnesium.
Chemical properties
It is monoclinic crystal, crystal form is clintheriform, columnar, aggregates shows dense block, fibers, flakes, earthy or kidney-shaped. It is glass shiny. It is slightly soluble in water and dissolved in hydrochloric acid.
Uses
(1) Calcium sulfate dihydrate can be used as raw material of building materials and cement, it can also be widely used in rubber, plastics, fertilizers, pesticides, paints, textiles, food, pharmaceutical, paper, household chemicals, arts and crafts, culture and education sectors. In the areas of absence of sulfur resource Calcium sulfate dihydrate can be used to manufacture sulfuric acid and ammonium sulfate. Colorless and transparent gypsum can be used for optical materials.
(2) Calcium sulfate dihydrate can be used as raw material of manufacturing cement, calcium sulfate hemihydrate and sulfuric acid. Paint and paper industry can be used as fillers. It can be used as fertilizer in agriculture which can reduce the alkalinity of the soil, improve soil properties. Food grade can be used as nutritional supplements (calcium to strengthen), coagulants, yeast food, the dough adjusting agents, chelating agents, it can also be used as enhancer of tomatoes, potatoes, canned organizations, hardener of brewing water, flavor-enhancer of wine, etc.
(3) Calcium sulfate dihydrate can be used as hygroscopic agent of analysis of trace nitrogen fertilizer production of carbon dioxide and carbon monoxide and carbon dioxide. It can be used in paint, artificial ivory, paint, paper, dyes, printing, metallurgy, water treatment.
Production method
When natural gypsum mine removes impurity, clay is calcined and then milled together can get it.
Sodium sulfate is added into by-product (calcium chloride) of ammonia and alkali method to product alkali, the reaction product is refined to get calcium sulfate dihydrate.
Byproduct of manufacturing organic acid. For example: The byproduct calcium oxalate is decomposed by sulfuric acid when oxalic acid is manufacturing, and then it is refined to get calcium sulfate dihydrate.
There are also open-pit mining to underground mining. The former is the open hillside, stepped mining; the latter mining method which employs the majority of the mine shaft development or inclined to open up, and room and pillar mining method, next is comprehensive mining method. Mining technological process sees "phosphate rock." The vast majority of gypsum mining is used hand-selected method, some mine production is without any ore sorting, extraetedore is mined mineral.
Category
Pesticide
Flammability hazard properties
Thermal decomposition can emit poisonous sulfur oxides fumes; it is one kind of nuisance dust.
Storage characteristics
Treasury should have ventilation and should be low-temperature drying .
Extinguishing agent
water
Professional standards
TWA 15 mg/m 3 (total dust).
Chemical Properties
monoclinic; hardness 1.5–2.0 Mohs; lumps or white powder(s); used in manufacturing portland cement, plaster of paris and artificial marble [KIR78] [MER06] [STR93]
Uses
Used in this determination of oxalates
Uses
Calcium Sulfate Dihydrate is obtained from gypsum, a naturally occurring compound. Also used in the preparation of calcium sulfate and calcium sulfoaluminate.
Definition
Gypsum: a monoclinic mineralform of hydrated calcium sulphate,CaSO4.2H2O. It occurs in five varieties:rock gypsum, which is oftenred stained and granular; gypsite, animpure earthy form occurring as asurface deposit; alabaster, a purefine-grained translucent form; satinspar, which is fibrous and silky; andselenite, which occurs as transparentcrystals in muds and clays. It is usedin the building industry and in themanufacture of cement, rubber,paper, and plaster of Paris.
Definition
A mineral that consists of calcium sulfate with water molecules attached, or the rock that consists primarily of this mineral.
Hazard
Nasal symptoms.
Agricultural Uses
Selenite, which is a salt of selenic acid (H2SeO3), and very similar to sulphite, is made by the oxidation of selenium by nitric acid. The low solubility of ironselenite complexes is responsible for the non-toxic level of selenium in plants growing on acid soils having a very high total selenium content. Plants absorb selenite, but generally to a lesser extent, than selenate, which is another form of selenium.
Safety Profile
Human systemic effects by inhalation: fibrosing alveolitis ('growth of fibrous tissue in the lung), unspecified respiratory system effects, and unspecified effects on the nose. Questionable carcinogen with experimental carcinogenic data. Long considered a nuisance dust (dependmg on silica content). When heated to decomposition it emits toxic fumes of SO,. See also CALCIUM SULFATE, CALCIUM COMPOUNDS, and SULFATES.
Purification Methods
It loses only part of its H2O at 100-150o (see below). It is soluble in H2O and very slowly soluble in glycerol. It is insoluble in most organic solvents.
Calcium sulfate dihydrate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
View Lastest Price from Calcium sulfate dihydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-18 | Calcium sulfate dihydrate
10101-41-4
|
US $499.00 / ton | 1ton | 99% | 3000tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-03-15 | Calcium sulfate dihydrate
10101-41-4
|
US $20.00-18.00 / kg | 500kg | 99.9 | 200tons | Shandong Juchuang Chemical Co., LTD | |
 |
2023-06-17 | Calcium sulfate dihydrate
10101-41-4
|
US $10.00 / kg | 1kg | 99.99% | 50000tons | Henan Bao Enluo International TradeCo.,LTD |
-

- Calcium sulfate dihydrate
10101-41-4
- US $499.00 / ton
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Calcium sulfate dihydrate
10101-41-4
- US $20.00-18.00 / kg
- 99.9
- Shandong Juchuang Chemical Co., LTD
-

- Calcium sulfate dihydrate
10101-41-4
- US $10.00 / kg
- 99.99%
- Henan Bao Enluo International TradeCo.,LTD