Gliclazide
- CAS No.
- 21187-98-4
- Chemical Name:
- Gliclazide
- Synonyms
- GLIMICRON;1-(3-azabicyclo(3.3.0)oct-3-yl)-3-(p-tolylsulfonyl)urea;Gliclazide analogue: N-[[(Hexahydrocyclopenta [c]pyrrol-2(1H)-yl)aMino]carbonyl]-2-Methyl benzenesulfonaMide;s852;se1702;S-1702;Diaprel;Glyzide;DIAMICRON;Glinormax
- CBNumber:
- CB5113462
- Molecular Formula:
- C15H21N3O3S
- Molecular Weight:
- 323.41
- MDL Number:
- MFCD00409893
- MOL File:
- 21187-98-4.mol
- MSDS File:
- SDS
| Melting point | 163-169 °C (lit.) |
|---|---|
| Density | 1.2205 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | 2-8°C |
| solubility | methylene chloride: soluble |
| form | powder |
| pka | 6.07±0.10(Predicted) |
| color | white |
| Merck | 14,4439 |
| InChIKey | BOVGTQGAOIONJV-UHFFFAOYSA-N |
| CAS DataBase Reference | 21187-98-4(CAS DataBase Reference) |
| FDA UNII | G4PX8C4HKV |
| NCI Drug Dictionary | gliclazide |
| ATC code | A10BB09 |
| EPA Substance Registry System | Benzenesulfonamide, N-[[(hexahydrocyclopenta[c]pyrrol-2(1H)-yl)amino]carbonyl]-4-methyl (21187-98-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 21-36/38-46-62-63 | |||||||||
| Safety Statements | 25-26-36/37-53-24/25 | |||||||||
| RIDADR | 3077 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | YT4500000 | |||||||||
| HS Code | 29350090 | |||||||||
| Toxicity | LD50 orally in mice: >3 g/kg (Duhault) | |||||||||
| NFPA 704 |
|
Gliclazide price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR1288 | Gliclazide Pharmaceutical Secondary Standard; Certified Reference Material | 21187-98-4 | 1g | $97.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | G2167 | Gliclazide powder, ≥98% | 21187-98-4 | 5g | $158 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP368 | Gliclazide British Pharmacopoeia (BP) Reference Standard | 21187-98-4 | 200MG | $269 | 2024-03-01 | Buy |
| TCI Chemical | G0381 | Gliclazide >98.5%(HPLC)(T) | 21187-98-4 | 5g | $66 | 2024-03-01 | Buy |
| Cayman Chemical | 25503 | Gliclazide ≥98% | 21187-98-4 | 1g | $32 | 2024-03-01 | Buy |
Gliclazide Chemical Properties,Uses,Production
Description
Gliclazide (21187-98-4) is an oral antihyperglycemic agent used for the treatment of diabetes mellitus type II. It belongs to the sulfonylurea class of insulin secretagogues, which stimulates β cells of the pancreas to release insulin. Gliclazide binds to the β cell sulfonyl urea receptor (SUR1), further blocking the ATP sensitive potassium channels. Therefore, the potassium efflux substantially decreases, causing depolarization of the β cells. Then the voltage-dependent calcium channels in the β cell are open, resulting in calmodulin activation, which in turn leads to exocytosis of insulin containing secretorty granules. Recent studies have also shown that gliclazide can effectiveimprove anti-oxidant status and nitric oxide-mediated vasodilation in Type 2 diabetes and protect pancreatic beta-cells from damage by hydrogen peroxide.
Hypoglycemic agents
Gliclazide, chemical name is 1-(hexahydrocyclopenta [c] pyrrole-2 (1H)-yl)-3-(4-methylphenyl) sulfonyl urea, is the second generation of sulfur ureide oral hypoglycemic agents, and it also has dual function of hypoglycemic and improving blood clotting. It not only can improve the metabolism of diabetic patients, but also can improve or delay the occurrence of diabetic vascular complications. Gliclazide was developed by the French SERVIER company, and listed in France as early as 1972. Its trade names are diamicron gliclazide, methanesulfonic bicyclic urea to g pancreas, methanesulfonic grid urea, glick that sa. It is mainly used for the treatment of the onset of diet and exercise alone control ineffective of adulthood, and no ketosis tendency of light, moderate the type II diabetes. It also can improve diabetic retinopathy and metabolic disorders, vascular function. It can be used with biguanide oral hypoglycemic drugs, and used with insulin to treat insulin-dependent diabetes mellitus, in which condition that insulin dosage can be reduced. 1980s, it began to be supplied into the Chinese market. Now there has been more than 130 countries worldwide that registered and sold.
Pharmacological effects
1. Hypoglycemic effect: gliclazide is the second-generation oral sulfonylurea hypoglycemic agents. Its role is more than ten times stronger than tolbutamide. Mechanism of action is to stimulate pancreatic β cells to release insulin, and then the high blood sugar drops. This may be because that sulfonylurea combines with β cell surface receptor, and increases the activation and simultaneously improves the sensitivity of outer periphery of the target tissue to insulin.
2. It can reduce platelet aggregation and adhesion, and prevent fibrin depositing in the microvasculature.
3. It can lower cholesterol savings, and reduce plasma concentrations of arterial triphosphate glycerides and fatty acids. The role of the three not only can treat diabetes metabolic disorders, but also prevent and treat complications like the development and progression of diabetes-blood vessels, retinal, renal disease.
Pharmacokinetics
The absorption of gliclazide is rapidly when it is taken orally. The plasma concentration peaks after two to six hours. Plasma protein binding rate is 94.2%. Τ1/2 is about 12 hours. Gliclazide is mainly used in the liver metabolism, and its metabolites has no hypoglycemic effect. 98% is excreted by kidney in less than 48 hours, and the content of unchanged drug in the urine is less than 5%.
Synthetic method
Cyclopentane ortho anhydride as raw materials, ammoniates to obtain cyclopentane phthalimide. It reacts to obtain azabicyclo through catalytic reduction by catalysts like LiAlH4, KBH4/ZnCl2 or black platinum. And then azabicyclo reacts to give N-3-azabicyclo [3, 3, O] octane hydrochloride by nitrosation, zinc reduction. Finally it reacts with toluene sulfonylurea to obtain gliclazide through condensation.

Figure 1 The synthetic route of gliclazide
Side effects
Occasional mild nausea, vomiting, abdominal pain, constipation, diarrhea, erythema, urticaria, thrombocytopenia, neutropenia, anemia. Most adverse reactions disappeared after withdrawal.
Contraindications
1. Forbidden for patients allergic to gliclazide or sulfonylureas, sulfa drugs.
2. Forbidden for patients with type 1 diabetes.
3. Forbidden for patients with diabetic pre-coma, diabetic ketoacidosis.
4. Forbidden for patients with severe liver and kidney dysfunction.
5. Forbidden for leukopenia patients.
6. Forbidden for patients with stress situations like coma, severe burns, infection, trauma and major surgery.
7. Forbidden for patients with pregnant and lactating women
Precautions
1. When patients with type 2 diabetes have infection, trauma, surgery, stress situations and ketoacidosis and hyperosmolar nonketotic diabetic coma, insulin therapy should be used instead.
2. When the overdose of gliclazide, eating too little or strenuous exercise, you should take attention to prevent hypoglycemia.
3. You must regularly check blood sugar, urine, and take eye examinations.
4. When gliclazide is combined with anticoagulants, you should have regular blood clotting check.
References
https://www.drugbank.ca/drugs/DB01120
https://en.wikipedia.org/wiki/Gliclazide
Fava, D, et al. "Gliclazide improves anti-oxidant status and nitric oxide-mediated vasodilation in Type 2 diabetes." Diabetic Medicine A Journal of the British Diabetic Association 19.9(2002):752.
Kimoto, K, et al. "Gliclazide protects pancreatic beta-cells from damage by hydrogen peroxide. " Biochemical & Biophysical Research Communications 303.1(2003):112-119.
Chemical Properties
White Cyrstalline Solid
Originator
Diamicron,Servier,France,1972
Uses
Gliclazide is an oral hypoglycemic agent used to treat non-insulin-dependent diabetes mellitus.Treatment of diabetes associated with obesity or vascular disease, for adults with type 2 diabetes.Diabetes is a chronic (long-lasting) health condition that affects how your body turns food into energy. Gliclazide reduces blood glucose levels by stimulating insulin secretion from the β-cells of the islets of Langerhans.
Definition
ChEBI: Gliclazide is a N-sulfonylurea. It has a role as a hypoglycemic agent, a radical scavenger and an insulin secretagogue.
Manufacturing Process
To a suspension containing 4.86 parts of 4-methylbenzenesulfonyl urethane (MP 80° to 82°C) and 36 parts of anhydrous toluene there are rapidly added 2.5 parts of N-amino-3-azabicyclo(3.3.0)octane (BP/18 mm = 86°C). The reaction mixture is heated under reflux for 1 hour. The resulting clear solution crystallizes on cooling. The crystals are filtered, washed with 2 parts of toluene, then recrystallized from anhydrous ethanol. There are obtained 3.8 parts of the desired product, MP 180° to 182°C.
Therapeutic Function
Oral hypoglycemic
General Description
Chemically, gliclazide, 1-(3-azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea (Diamicron), isvery similar to tolbutamide, with the exception of the bicyclicheterocyclic ring found in gliclazide. The pyrrolidineincreases its lipophilicity over that of tolbutamide,which increases its half-life. Even so, the p-methyl is susceptibleto the same oxidative metabolic fate as observedfor tolbutamide, namely, it will be metabolized to a carboxylicacid.
Biochem/physiol Actions
Oxidative modification of low-density lipoprotein (LDL) plays an important role in vascular dysfunction associated with diabetes mellitus. Gliclazide is a second-generation sulfonylurea with free-radical-scavenging activity. Incubation of human aortic smooth muscle cell (HASMC) with native human LDL (100 μg/mL) in the presence of increasing concentrations of gliclazide (1 to 10 μg/mL) resulted in a dose-dependent decrease in HASMC-mediated LDL oxidation. Exposure of HASMCs to gliclazide (1 to 10 μg/mL) and native LDL (100 μg/mL) also led to a dose-dependent decrease in oxidized LDL-induced human monocyte adhesion to HASMCs. In addition, incubation of HASMCs with gliclazide dramatically reduced the ability of oxidized LDL to stimulate the proliferation of these cells. Finally, treatment of HASMCs with gliclazide resulted in a marked decrease in oxidatively modified LDL-induced monocyte chemoattractant protein (MCP)-1 and human heat shock protein 70 (HSP 70) expression, both at the gene and protein levels. These results show that gliclazide, at concentrations in the therapeutic range (5 to 10 μg/mL), is effective in vitro in reducing vascular smooth muscle cell (VSMC) dysfunction induced by oxidatively modified LDL. Administration of gliclazide to type 2 diabetic patients could form part of the strategy for the prevention and management of diabetic cardiovascular diseases
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs.
Antibacterials: effects enhanced by chloramphenicol,
sulphonamides, tetracyclines and trimethoprim;
effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by
coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole
and miconazole and possibly voriconazole - avoid
with miconazole.
Lipid-regulating drugs: possibly additive
hypoglycaemic effect with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas.
When gliclazide is used with nonsteroidal anti-inflammatory drug (especially salicylates), sulfa antibiotic, double coumarin anticoagulants, monoamine oxidase inhibitors, β-blockers, tetracycline, chloramphenicol, dicyclohexyl B piperidine, clofibrate, ethanol and other drugs, its dosage should be reduced to avoid hypoglycemia reaction.
Metabolism
Gliclazide is extensively metabolised in the liver to
metabolites that have no significant hypoglycaemic
activity.
Metabolites and a small amount of unchanged drug are
excreted in the urine.
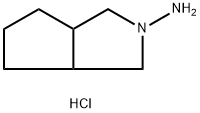
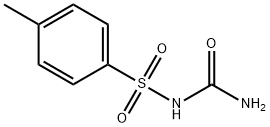
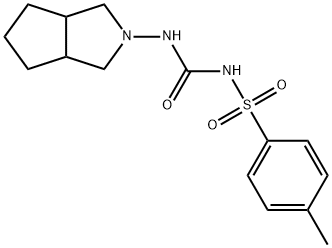
Gliclazide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Kindchem(Nanjing)Co.,Ltd | +86-025-025-85281586 +8618651653755 | sales@kindchem.cn | China | 1227 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 864 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
Related articles
- Gliclazide: Pharmacodynamic Properties, Pharmacokinetic Properties and Therapeutic Efficacy
- Gliclazide effectively reduces blood glucose levels in non-insulin dependent diabetes mellitus patients by improving insulin s....
- Feb 21,2024
- A sulfonylurea oral hypoglycemic agent: Gliclazide
- Gliclazide is a second-generation, sulfonylurea oral hypoglycemic agent. It can improve blood clotting and postpone the compli....
- Nov 9,2023
View Lastest Price from Gliclazide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Gliclazide
21187-98-4
|
US $125.00-65.00 / Kg/Bag | 1Kg/Bag | BP2017 | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-05 | Gliclazide
21187-98-4
|
US $0.00 / kg | 1kg | 99% | 20tons | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-01-02 | Gliclazide
21187-98-4
|
US $0.00-0.00 / kg | 1kg | 98% | 100 | Kindchem(Nanjing)Co.,Ltd |
-

- Gliclazide
21187-98-4
- US $125.00-65.00 / Kg/Bag
- BP2017
- Sinoway Industrial co., ltd.
-

- Gliclazide
21187-98-4
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Gliclazide
21187-98-4
- US $0.00-0.00 / kg
- 98%
- Kindchem(Nanjing)Co.,Ltd
21187-98-4(Gliclazide)Related Search:
1of4





