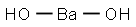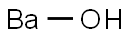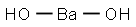Barium hydroxide
- CAS No.
- 17194-00-2
- Chemical Name:
- Barium hydroxide
- Synonyms
- Ba(OH)2;Anhydrous Barium hydroxide;Aetzbaryt;Chebi:32592;BARIUM HYDRATE;baryta hydrate;Bariumhydroxid;BARIUM HYDROXIDE;bariumdihydroxide;Barium dihydoxide
- CBNumber:
- CB5118700
- Molecular Formula:
- BaH2O2
Lewis structure
2.png)
- Molecular Weight:
- 171.34
- MDL Number:
- MFCD00003443
- MOL File:
- 17194-00-2.mol
- MSDS File:
- SDS
| Melting point | >300 °C(lit.) |
|---|---|
| Boiling point | decomposes at 1032 (calculated) [KNA91] |
| Density | 2.2 g/mL at 25 °C(lit.) |
| solubility | Aqueous Acid (Slightly), Water (Slightly) |
| form | Solid |
| color | Clear |
| PH | 11.27(1 mM solution);12.22(10 mM solution);13.08(100 mM solution) |
| Odor | no odor |
| Water Solubility | Soluble in water. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,977 |
| Exposure limits |
ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability | Stable. Incompatible with acids, carbon dioxide, moisture. |
| InChIKey | RQPZNWPYLFFXCP-UHFFFAOYSA-L |
| CAS DataBase Reference | 17194-00-2(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | BARIUM HYDROXIDE |
| FDA 21 CFR | 177.2410 |
| EWG's Food Scores | 1 |
| FDA UNII | 1OHB71MYBK |
| NIST Chemistry Reference | Barium dihydroxide(17194-00-2) |
| EPA Substance Registry System | Barium hydroxide (Ba(OH)2) (17194-00-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H332-H314 | |||||||||
| Precautionary statements | P260-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi,Xn | |||||||||
| Risk Statements | 36/37/38-34-20/22-36/38-41-35-22 | |||||||||
| Safety Statements | 26-36/37/39-45-36/37 | |||||||||
| RIDADR | UN 3262 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | CQ9200000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2805191000 | |||||||||
| Toxicity | LD50 ipr-mus: 255 mg/kg GTPZAB 28(6),45,84 | |||||||||
| NFPA 704 |
|
Barium hydroxide price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 433373 | Barium hydroxide technical grade, ~95% | 17194-00-2 | 250g | $49 | 2024-03-01 | Buy |
| Sigma-Aldrich | 433373 | Barium hydroxide technical grade, ~95% | 17194-00-2 | 1kg | $116 | 2024-03-01 | Buy |
| Alfa Aesar | 012195 | Barium hydroxide, anhydrous, 94-98% | 17194-00-2 | 50g | $26.5 | 2023-06-20 | Buy |
| Alfa Aesar | 012195 | Barium hydroxide, anhydrous, 94-98% | 17194-00-2 | 1kg | $98.8 | 2023-06-20 | Buy |
| Strem Chemicals | 93-5616 | Barium hydroxide, anhydrous, min. 95% | 17194-00-2 | 100g | $34 | 2024-03-01 | Buy |
Barium hydroxide Chemical Properties,Uses,Production
Description
Barium hydroxide is a kind of barium base. It has many kinds of applications. It can be used as the precursor for the preparation of other barium compounds. Its monohydrate form can be used to dehydrate and remove sulfate in many products. In laboratory, it can be used in analytic chemistry for the titration assay of organic acid. In organic synthesis, it is a strong base used for the hydrolysis of esters and nitriles. It is also a useful catalyst of organic synthesis. Other applications also include acting as an interlayer between zinc oxide and a luminescent conjugated polymer for light-emitting diodes, extracting pure arabinoxylans from water-insoluble cell wall material of wheat flour, removing sulfate and metals from the water and acting as a selective precipitating agent for hemicelluloses.
Chemical Properties
barium hydroxide is a white or colorless, poisonous powder or crystalline substance, Ba(OH)2, used for example, in the saponification of fats and the fusing of silicates. Barium hydroxide forms a strong caustic base in aqueous solution. It has many uses, e.g., as a test for sulfides; in pesticides; in the manufacture of alkali and glass.

barium hydroxide, anhydrous. the commercially available preparation usually contains 92-95% of the monohydrate and is poisonous. barium hydroxide, monohydrate (barium monohydrate). a white, poisonous, slightly water-soluble, powder, Ba(OH)2.H2O; it is soluble in dilute acids. barium hydroxide, octahydrate is a poisonous white powder, Ba(OH)2.8H2O, that is soluble in water, ethanol, and ether; it absorbs CO2 from the atmosphere. It is used chiefly in organic preparations and analytical chemistry. barium hydroxide,pentahydrate. toxic white fakes, Ba(OH)2.5H2O; uses are the same as those of the octahydrate.
Uses
Barium hydroxide, especially the monohydrate, is used to produce organic barium compounds such as additives for oil and stabilizers for plastics. In addition, barium hydroxide is used for. dehydration and deacidification, especially for removing sulfuric acid from fats, oils, waxes, and glycerol. In some applications, e.g., removal of sulfate from water and as starting material for other barium compounds, barium hydroxide to some ex1ent replaces barium carbonate. The earlier use of the hydroxide in some countries forextracting the residual sucrose from molasses is no longer of much significance. The re- cent patent literature describes a procedure in which barium hydroxide is reacted with hydrogen peroxide to produce barium peroxide monohydrate.
Production Methods
The anhydrous hydroxide has only a secondary industrial importance; the monohydrate and octahydrate are used in industry on a far larger scale.
Barium hydroxide octahydrate is produced by hydration of barium oxide. The barium oxide is suspended in recycled mother lye and heated for several hoursto destroy peroxides. The insolubles, e.g., BaC03 , are separated, and the hydroxide is precipitated by crystallization at low temperatures in stainless steel vessels. The crystals are removed from the mother lye by centrifugation.
Another industrial method is based on the oxidation of a barium sulfide solution. Air is passed into the stirred solution containing barium hydroxide and barium hydrosulfide, the hydrolysis products of barium sulfide, until the hydrosulfide is oxidized to form polysulfide. Then the barium hydroxide octahydrate is crystallized atlow temperatures and separated with a centrifuge. The Polysulfide mother lye is partly recycled, the rest usually processed for other barium compounds.
The monohydrate is generally produced on a commercial scale by dehydration of the octahydrate in heated vacuum driers. A monohydrate of especially high reactivity is obtained with drum driers. This highly reactive monohydrate is particularly suitable for the production of oil additives.
References
[1]Wu, Mingmei, et al. "Hydrothermal Synthesis of Tetragonal Barium Titanate from Barium Hydroxide and Titanium Dioxide under Moderate Conditions." Journal of the American Ceramic Society 82.11 (2010):3254-3256.
[2]Meier, Hans, et al. "Barium Hydroxide as a Selective Precipitating Agent for Hemicelluloses." Acta Chemica Scandinavica 12.1(1958):144-146.
[3]Gruppen, H., R. J. Hamer, and A. G. J. Voragen. "Barium hydroxide as a tool to extract pure arabinoxylans from water- insoluble cell wall material of wheat flour." Journal of Cereal Science 13.3(1991):275-290.
[4]Li, Ping Lu, D. Kabra, and R. H. Friend. "Barium Hydroxide as an Interlayer Between Zinc Oxide and a Luminescent Conjugated Polymer for Light-Emitting Diodes." Advanced Functional Materials 22.19(2012):4165–4171.
[5]Bologo, V., J. P. Maree, and F. Carlsson. "Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water." Water Sa 38.1(2012):23-28.
[6]Sinisterra, J. V., A. Fuentes, and J. M. Marinas. "Barium hydroxide as catalyst in organic reactions. 17. Interfacial solid- liquid Wittig-Horner reaction under sonochemical conditions." J.org.chem (1987).
Description
Barium Hydroxide occurs as an anhydrate whose prismatic, colorless
crystals are very deliquescent. Barium hydroxide can
be prepared by dissolving barium oxide (BaO) in water:
BaO+ 9H2O→Ba(OH)2·8H2O
It crystallizes as the octahydrate, which converts to
the monohydrate upon heating in air. Barium oxide
has been found to react with water vapor at temperatures
from 1443 to 1593 K to form gaseous Ba(OH)2
according to the reaction:
BaO(solid)+H2O(gas)→Ba(OH)2(gas)
A better method of preparation involves addition of
NaOH to the nitrate. Since barium hydroxide is fairly
soluble, it is necessary to evaporate the solution to crystallize Ba(OH)2·8H2O. Ethanol is added to aid in this crystallization. The crystals are washed in cold ethanol to remove sodium ions and then dried.
Chemical Properties
Barium hydroxide,Ba(OH)2·8H20, is a white crystalline powder, colorless solid composed of monoclinic crystals. It has a melting point of 78°C and is soluble in water and insoluble in acetone. It is used in the fusion of silicates and fat saponification.
Physical properties
Monohydrate, Ba(OH)2?H2O is a white powder; density 3.743 g/cm3; slightly soluble in water; soluble in dilute mineral acids. Octahydrate, Ba(OH)2?8H2O is a colorless monoclinic crystal; density 2.18 g/cm3 at 16°C; refractive index 1.50; melts at 78°C; vapor pressure 227 torr; loses seven molecules of water of crystallization when its solution is boiled in the absence of atmospheric CO2 forming solid monohydrate; further heating produces anhydrous Ba(OH)2 melting at 407°C; readily dissolves in water (3.76 g/100 g at 20°C and 11.7 g/100 g at 50°C); aqueous solution highly alkaline; also soluble in methanol; slightly soluble in ethanol; insoluble in acetone.
Uses
Carbon dioxide absorbant.
Uses
Barium hydroxide is used in analytical chemistry to titrate weak acids, particularly organic acids. It forms clear aqueous solutions that are free from carbonate, unlike those of the alkali hydroxides, since barium carbonate is insoluble in water. This allows the use of indicators such as phenolphthalein or thymophthalein (with alkaline color changes) without the risk of titration errors due to the presence of weakly basic CO32- ions.Barium hydroxide is used in organic synthesis as a strong base, for example for the hydrolysis of esters and nitriles.It has been used to hydrolyze one of the two equivalent ester groups in dimethyl endecanedioate. It is also used in the preparation of cyclopentanone, diacetone alcohol and d-Gluonic g-lactone.
Barium hydroxide is used as an additive in thermoplastics (such as phenolic resins), rayon and PVC stabilizers to improve plastic properties. This material is used as a general-purpose additive for lubricants and greases. Other industrial applications for barium hydroxide include sugar fabrication, manufacturing soaps, fat saponification, fusing of silicates and chemical synthesis of other barium compounds and organic compounds.
Barium hydroxide is offered for sale both as the octahydrate and the monohydrate. It is available in large quantities commercially.
Uses
Bariium hydroxide is used as a precursor to other barium compounds. It is used in analytical chemistry for titration of weak acids, particularly organic acids. It removes the carbonate in water. It is a strong base, and is used as catalyst in many organic reactions, such as hydrolysis of esters and nitriles, decarboxylation of amino acids, selective hydrolysis of one of the ester groups in dimethyl hendecanedioate, and preparation of cyclopentanone, diacetone alcohol and D-Gulonic gamma-lactone.
Preparation
Barium hydroxide is made by dissolving barium oxide in hot water. The octahydrate, Ba(OH)2?8H2O, crystallizes upon cooling. It also is prepared by precipitation with caustic soda from an aqueous solution of barium sulfide:
BaS + 2NaOH → Ba(OH)2 + Na2S.
Hazard
An acute poison; toxic symptoms are similar to other soluble salts of barium (see Barium).
Flammability and Explosibility
Not classified
Safety Profile
A poison by ingestion andintraperitoneal routes. Incompatible with chlorinated rubber.
Barium hydroxide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
Related Qustion
- Q:Is Ba(OH)2 a strong base?
- A:As the metal it is made of (barium) sits at the bottom of Group II, its hydroxide is considerably strong and comparable to Gro....
- Mar 21,2024
View Lastest Price from Barium hydroxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Barium hydroxide
17194-00-2
|
US $0.60 / kg | 10kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2023-11-10 | Barium hydroxide
17194-00-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-09-07 | Barium hydroxide
17194-00-2
|
US $0.00-0.00 / kg | 1kg | 0.99 | 100tons | Hebei Yanxi Chemical Co., Ltd. |
-

- Barium hydroxide
17194-00-2
- US $0.60 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Barium hydroxide
17194-00-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Barium hydroxide
17194-00-2
- US $0.00-0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.