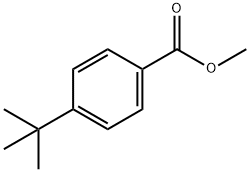
Methyl 4-tert-butylbenzoate
- CAS No.
- 26537-19-9
- Chemical Name:
- Methyl 4-tert-butylbenzoate
- Synonyms
- RARECHEM AL BF 0269;TIMTEC-BB SBB000305;METHYL 4-T-BUTYLBENZOATE;Methyl 4-tert-butylbenzoat;Methyl p-tert-butylbenzoate;butylbenzoicacidmethylester;METHYL 4-TERT-BUTYLBENZOATE;Methyl 4-Tert-Buthylbenzoate;Methyl4-tert-Butylbenzoate>4-tert-Butylbenzoic acid methyl
- CBNumber:
- CB5134874
- Molecular Formula:
- C12H16O2
- Molecular Weight:
- 192.25
- MDL Number:
- MFCD00008835
- MOL File:
- 26537-19-9.mol
- MSDS File:
- SDS
| Boiling point | 122-124 °C9 mm Hg(lit.) |
|---|---|
| Density | 0.995 g/mL at 25 °C(lit.) |
| vapor pressure | 1.36hPa at 20℃ |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| form | Liquid |
| Specific Gravity | 0.995 |
| color | Clear colorless to yellow |
| Water Solubility | 35mg/L at 20℃ |
| InChIKey | UPIJOAFHOIWPLT-UHFFFAOYSA-N |
| LogP | 4.3 at 25℃ |
| CAS DataBase Reference | 26537-19-9(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8VK4O27JMT |
| NIST Chemistry Reference | 4-Tert-butylbenzoic acid methyl ester(26537-19-9) |
| EPA Substance Registry System | Benzoic acid, 4-(1,1-dimethylethyl)-, methyl ester (26537-19-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332 | |||||||||
| Precautionary statements | P280-P301+P312+P330-P302+P352+P312-P304+P340+P312 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29163990 | |||||||||
| NFPA 704 |
|
Methyl 4-tert-butylbenzoate price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 167533 | Methyl 4-tert-butylbenzoate 99% | 26537-19-9 | 25g | $192 | 2023-06-20 | Buy |
| TCI Chemical | M1693 | Methyl 4-tert-Butylbenzoate >98.0%(GC) | 26537-19-9 | 25g | $17 | 2024-03-01 | Buy |
| TCI Chemical | M1693 | Methyl 4-tert-Butylbenzoate >98.0%(GC) | 26537-19-9 | 500g | $115 | 2024-03-01 | Buy |
| Alfa Aesar | A18619 | Methyl 4-tert-butylbenzoate, 98+% | 26537-19-9 | 25g | $23.65 | 2024-03-01 | Buy |
| Alfa Aesar | A18619 | Methyl 4-tert-butylbenzoate, 98+% | 26537-19-9 | 100g | $32.65 | 2024-03-01 | Buy |
Methyl 4-tert-butylbenzoate Chemical Properties,Uses,Production
Overview
Methyl 4-tert-butylbenzoate appears as white crystalline powder, being slightly soluble in water with the water solubility of 0.006g/100ml Water at 20 ° C. It can be miscible in ethanol, ether and other organic solvents with the organic toluene solubility of 9.0%, being an important organic synthesis intermediates. It is an important additive during the production of PVC heat stabilizer, PP nucleating agent, sunscreen and scaling powder. As the alkyd resin modifier, it can improve the resin luster, color, and speed up the resin drying time and improve the chemical resistance of the performance. The ammonium salt can improve the performance of friction parts and prevent rust, thus can be used as the additives of cutting oil and lubricants. Its sodium salt, barium salt, zinc salt can be used as polymer stabilizer and nucleating agent.
Synthesis method
The synthesis method of P-tert-butyl benzoic acid includes:
The oxidation method of liquid phase solvent, take acetic acid as solvent, lead acetate as oxidant and synthesize p-tert-butyl benzoic acid at low temperature.
Liquid non-solvent oxidation method: there is no need of any solvent; take cobalt acetate and sodium bromide as a catalyst and apply air oxidation at 170e temperature to obtain the final products.
High temperature gas phase oxidation method, in the presence of catalyst, high temperature lead to vaporization and oxidation of p-tert-butyl toluene to generate p-tert-butyl benzoic acid.
Recently, we found that there is no need of any catalyst if using cheap nitric acid as the oxidant for oxidation of p-tert-butyl toluene to get the crude t-butyl benzoic acid, followed by being dissolved with sodium hydroxide to remove organic impurities and insoluble matter, and then being acidified to obtain pure p-tert-butyl benzoic acid. This method has good selectivity and low energy consumption, and can avoid the shortcomings of air oxidation method such as low oxidation conversion rate, thus having great practical value.
Preparation of crude p-tert-butyl benzoic acid:
To a 500 mL autoclave, add 26 mL (0.2 mol, 29.6 g) of p-tert-butyltoluene, 26 mL 68% nitric acid (0.4 mol, 37 g) and 2.5 mL of water. The reaction stirrer was started and the temperature was raised to 180e. The reaction lasted for 8 hour followed by cooling with cool water. Open the reactor with most of the solid being deposited on the bottom of the reactor and attached to the reactor cooling tube in a small amount. The solid was collected, filtered and dried to give 34.5 g with a yield of 95.5%.
The purification of P-tert-butylbenzoic acid
Weigh 10 g of sodium hydroxide and add 90 mL of water to prepare a 10% sodium hydroxide solution. Add the above solid into it. After being sufficiently dissolved, the filtrate was adjusted to pH 3 with hydrochloric acid, and a large amount of crystals would be precipitated at this time. The solution was allowed to stand for 33.4 g with a yield of 98.2%. The product was analyzed by infrared spectroscopy, displaying the characteristic peaks of p-tert-butylbenzoic acid. The content was 98.4% by HPLC analysis with a melting point of 164~166e (literature values 164~165e).
The effect of nitric acid concentration, reaction temperature and reaction time on the synthesis of products
- Effect of nitric acid concentration on the product
- The effect of reaction temperature on the product
- The effect of reaction time on the reaction
From the analysis of the data, we can see that the reaction temperature has the greatest influence on the yield. Considering various factors, we selected the reaction conditions as: the temperature: 180e, reaction time: 8h and nitric acid concentration: 10%. According to this condition, the content of p-tert-butylbenzoic acid was 98.4% and the yield was 95.5%.
Application
Important intermediates for organic synthesis.
Chemical Properties
CLEAR COLOURLESS TO YELLOW LIQUID
Uses
Methyl 4-tert-butylbenzoate was used in the synthesis of tris(4-tert-butylphenyl)methyl chloride.
Synthesis Reference(s)
Tetrahedron, 42, p. 553, 1986 DOI: 10.1016/S0040-4020(01)87454-8
General Description
Methyl 4-tert-butylbenzoate undergoes Claisen condensation reaction with 4-methoxyacetophenone to give avobenzone, an ingredient of sunscreen products.
Methyl 4-tert-butylbenzoate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Methyl 4-tert-butylbenzoate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-22 | Methyl 4-tert-butylbenzoate
26537-19-9
|
US $30.00 / kg | 1kg | 99% | 1000t/year | Anhui Ruihan Technology Co., Ltd | |
 |
2023-08-18 | Methyl 4-tert-butylbenzoate
26537-19-9
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-06-29 | Methyl 4-tert-butylbenzoate
26537-19-9
|
US $0.00-0.00 / kg | 1kg | 99% | 50000kg | Hebei Yibangte Import and Export Co. , Ltd. |
-

- Methyl 4-tert-butylbenzoate
26537-19-9
- US $30.00 / kg
- 99%
- Anhui Ruihan Technology Co., Ltd
-

- Methyl 4-tert-butylbenzoate
26537-19-9
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Methyl 4-tert-butylbenzoate
26537-19-9
- US $0.00-0.00 / kg
- 99%
- Hebei Yibangte Import and Export Co. , Ltd.