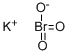Potassium bromate
- CAS No.
- 7758-01-2
- Chemical Name:
- Potassium bromate
- Synonyms
- eecno.e924;ACS,≥99.8%;ACS, 99.8% min;POTASSIUM BROMATE;potassium bronlate;Potassiumbromate,99%;POTASSIUM BROMATE PURE;POTASSIUM BROMATE, ACS;Potassium bromate, p.a.;Potassium bromate, 99.5%
- CBNumber:
- CB5281569
- Molecular Formula:
- BrKO3
- Molecular Weight:
- 167
- MDL Number:
- MFCD00011359
- MOL File:
- 7758-01-2.mol
- MSDS File:
- SDS
| Melting point | 350 °C |
|---|---|
| Density | 3.27 |
| storage temp. | Store below +30°C. |
| solubility | Potassium bromate is highly soluble in water (7.5 g/100 mL at 25°C; 49.8 g/100 mL at 100°C), slightly soluble in ethanol, and almost insoluble in acetone; it is very stable when dissolved in water at room temperature. |
| form | Powder/Solid |
| Specific Gravity | 3.27 |
| color | White crystals or granules |
| PH | 5.0-9.0 (25℃, 50mg/mL in H2O) |
| Water Solubility | 70 g/L (20 ºC) |
| Merck | 14,7617 |
| Stability | Strong oxidizer - contact with combustible materials may cause fire. Incompatible with combustible material, organics, reducing agents, aluminium, finely powdered metals. |
| FDA 21 CFR | 172.730 |
| Substances Added to Food (formerly EAFUS) | POTASSIUM BROMATE |
| CAS DataBase Reference | 7758-01-2(CAS DataBase Reference) |
| EWG's Food Scores | 6-9 |
| FDA UNII | 04MB35W6ZA |
| Proposition 65 List | Potassium bromate |
| IARC | 2B (Vol. Sup 7, 73) 1999 |
| EPA Substance Registry System | Potassium bromate (7758-01-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H301-H350 | |||||||||
| Precautionary statements | P201-P210-P301+P310+P330 | |||||||||
| Hazard Codes | O,T | |||||||||
| Risk Statements | 45-9-25-22 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | UN 1484 5.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EF8725000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28299000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 157 mg/kg | |||||||||
| NFPA 704 |
|
Potassium bromate price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 309087 | Potassium bromate ACS reagent, ≥99.8% | 7758-01-2 | 5g | $38.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 309087 | Potassium bromate ACS reagent, ≥99.8% | 7758-01-2 | 100g | $129 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.60308 | Potassium bromate solution 1/60?M KBrO3 (0.1 N), Titripur? | 7758-01-2 | 1L | $83 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04912 | Potassium bromate for analysis (max 0,000001% Hg) EMSURE? ACS,ISO,Reag. Ph Eur | 7758-01-2 | 100g | $88.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04912 | Potassium bromate for analysis (max 0,000001% Hg) EMSURE? ACS,ISO,Reag. Ph Eur | 7758-01-2 | 250g | $144 | 2024-03-01 | Buy |
Potassium bromate Chemical Properties,Uses,Production
Description
Potassium bromate is the potassium salt of bromate. It is typically used in the United States as a flour improver. It acts to strengthen the dough and to allow higher rising. Potassium bromate (KBrO3) is an oxidizing agent that has been used as a food additive, mainly in the bread-making process. However, it has been realized that it is a potential carcinogenic agent. The agent is carcinogenic in rats and nephrotoxic in both man and experimental animals when given orally. It has been demonstrated that KBrO3 induces renal cell tumors, mesotheliomas of the peritoneum, and follicular cell tumors of the thyroid. In addition, experiments aimed at elucidating the mode of carcinogenic action have revealed that KBrO3 is a complete carcinogen, possessing both initiating and promoting activities for rat renal tumorigenesis.
Chemical Properties
Potassium bromate is a white crystal, granules or powder, which is colourless, odourless, and tasteless. It has no medicinal value but is added to flour as a maturing agent, to dough, to fish paste as a conditioner, and also to beer or cheese (Chipman 1988). It has also been used as a constituent in cold wave hair solution (Ueno 2000).

Potassium bromate is a substance that has a vapour density of 5.8 (air=1) and density of 3.27 (gcm3 ) and when in contact with combustible material may cause fire. It is incompatible with organics, reducing agents, aluminum, and finely powdered metals (USEPA 1993). When heated, it produces toxic fumes of bromine (RCC 1999).It has an infinite solubility in water, and at drinking water pH, it should exist almost exclusively in the ionic form (USEPA 1998a). The problem of potassium bromate started with ozonation of drinking water to form bromate as a major by product (WHO (1993). When research was done to confirm the safety of ozonated water, it was found that potassium bromate causes renal cancer in rats when they drank water with potassium bromate.Following this discoveries, many countries, Health Organizations and Agencies started banning the use of potassium bromate (NAFDAC ,2003). Some of the countries where potassium bromate has been banned include United Kingdom in 1990 and Canada in 1994. Other countries where potassium bromate has been removed from the list of permitted food additives are Belgium, Greece, Norway, Denmark, Spain, Portugal, Japan, and Switzerland (NAFDAC 2003). WHO also banned the use of potassium bromate in 1993.
Uses
Potassium bromate (KBrO3) is used primarily as a maturing agent for flour and as a dough conditioner. It is recognised as one of the best dough conditioners in the bakery industry. Scientific evidence surfaced in 1990, implicating bromate as a possible carcinogen. In response to the potential hazards, the United Nations Joint Food and Agricultural Organisation and the World Heath Organisation decided to revoke potassium bromide from the list of acceptable flour treatment additives.
As nonfood usage, KBrO3 has been introduced as an oxidizing agent, a primary standard, and a brominating agent in analytical chemistry. Its oxidizing property has further been used in home permanent-wave neutralizing compounds at concentrations of between 5 to 25% at pH 4 to 9, together with sodium bromate, sodium perborate, or hydrogen peroxide.
Preparation
Potassium bromate can be produced by passing bromine through a solution of potassium hydroxide. However, the compound is manufactured mainly by large-scale industrial electrolytic processes.
Toxicity and carcinogenicity
Potassium bromate had been used as a flour treatment agent for over 80 years. It is a powerful oxidising agent and it became known that it is carcinogenic.
Initially, potassium bromate was assumed to react completely on baking. However, testing revealed that traces of it survived in to the finished product.
After 1990 the British government changed the law to remove potas- sium bromate from bread. This move was logical in the circumstances. It was convenient because most of the EU did not permit potassium bromate and its continued use in British four was a bar to enter EU trade.
Food Safety and Standards Authority of India (FSSAI) has directed manufacturers of food products to stop using potassium bromate as an additive. The country’s apex food regulator clarified that its use as an additive in food products will not be permitted under any circumstances.
Only the United States Food and Drug Administration (USFDA) permits the use of potassium bromate. It is also used by flour mills for refined flour. But as per as USFDA rules, the use of potassium bromate is allowed in a prescribed limit, i e 0.0075 part for each 100 parts by weight of flour used.
Potassium bromate is classified as a Category 2B carcinogen. It is included in the World Health Organisation’s (WHO) list of ingredients causing cancer.
References
[1]Ekop, A. S., I. B. Obot, and E. N. Ikpatt. "Anti-Nutritional Factors and Potassium Bromate Content in Bread and Flour Samples in Uyo Metropolis, Nigeria." Journal of Chemistry 5.4(2008):736-741.
[2]Kurokawa, Y, et al. "Carcinogenicity of potassium bromate administered orally to F344 rats. " Journal of the National Cancer Institute 71.5(1983):965-72.
[3]Kurokawa, Y, et al. "Toxicity and carcinogenicity of potassium bromate--a new renal carcinogen. " Environmental Health Perspectives 87.1(1990):309.
Chemical Properties
Potassium bromate is a white crystalline solid.
Uses
Potassium Bromate is a dough conditioner that exists as white crystals or powder and is soluble in water. it exists in the anhydrous form as white granular powder and in the hydrated form as small white crystals or granules. it is used to age and improve the baking properties of flour. it is used with potassium iodate and azodicarbon- amide to modify the protein in bread flour to promote the desired properties of loaf volume and shape. it is used in baked goods.
Uses
It is used in oxidizing agent in acid solutions.
Uses
Bread- and flour-improving agent; in analytical chemistry.
Definition
ChEBI: Potassium bromate is a potassium salt and a bromate salt. It has a role as a flour treatment agent.
General Description
A white crystalline solid.
Air & Water Reactions
Water soluble.
Reactivity Profile
Potassium bromate is a strong oxidizing agent. Forms very flammable mixtures with combustible materials. Such mixtures may be explosive if the combustible material is finely divided and are often ignitable by friction. A mixture with finely divided aluminum can explode by heat, percussion, and friction [Mellor 2:310. 1946-47]. Reacts explosively with selenium [Mellor 2 Supp1:763. 1956]. Prolonged exposure to fire or heat can result in an explosion .
Hazard
Dangerous fire risk in contact with organic materials, strong irritant. Possible carcinogen.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. A poison by ingestion. A powerful oxidzer. An irritant to skin, eyes, and mucous membranes. Mutation data reported. Wxtures with sulfur may ignite. Violent reaction with Al, Al + dmitrotoluene @ 290°, As, C, Cu, Pb(C2H3O2)2, metal sulfides, organic matter, P, S. Aqueous solutions react violently with selenium. When heated to decomposition it emits very toxic fumes of Brand K2O. See also BROMIDES.
Potential Exposure
Potassium bromate is used as animal feed additive, food additive; flavor and packaging material; as a laboratory reagent; an oxidizing agent.
Shipping
UN1479 Potassium bromate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
Crystallise KBrO3 from distilled H2O (2mL/g) between 100o and 0o. To remove bromide contamination, a 5% solution in distilled H2O, cooled to 10o, is bubbled with gaseous chlorine for 2hours, then filtered and extracted with reagent grade CCl4 until colourless and odourless. After evaporating the aqueous phase to about half its volume, it is cooled again slowly to about 10o. The crystalline KBrO3 that separates, is washed with 95% EtOH and dried in a vacuum [Boyd et al. J Am Chem Soc 74 237 1952]. Another way to remove Br-ions is by stirring several times in MeOH and then drying at 150o [Field & Boyd J Phys Chem 89 3767 1985].
Incompatibilities
A strong oxidizer.Violent reaction with many compounds, including reducing agents; chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/ friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide. Incompatible with aluminum, copper.
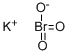
Potassium bromate Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | cherry@crovellbio.com | China | 18457 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 | sales8@anhuiyiao.com | China | 253 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
View Lastest Price from Potassium bromate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Potassium bromate
7758-01-2
|
US $3.00 / kg | 10kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2023-11-02 | Potassium bromate
7758-01-2
|
US $0.00 / kg | 1kg | 99% | 1 tons | Anhui Yiao New Material Technology Co., Ltd | |
 |
2023-11-02 | Potassium bromate
7758-01-2
|
US $0.00 / kg | 1kg | 99% | 1 tons | Anhui Yiao New Material Technology Co., Ltd |
-

- Potassium bromate
7758-01-2
- US $3.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Potassium bromate
7758-01-2
- US $0.00 / kg
- 99%
- Anhui Yiao New Material Technology Co., Ltd
-

- Potassium bromate
7758-01-2
- US $0.00 / kg
- 99%
- Anhui Yiao New Material Technology Co., Ltd