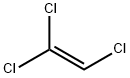
Trichloroethylene
- CAS No.
- 79-01-6
- Chemical Name:
- Trichloroethylene
- Synonyms
- TCE;TRI;TRICHLORETHYLENE;TRICHLOROETHENE;1,1,2-TRICHLOROETHENE;1979/1/6;Triol;C2HCl3;Trichlorethylen;Triad
- CBNumber:
- CB5406573
- Molecular Formula:
- C2HCl3
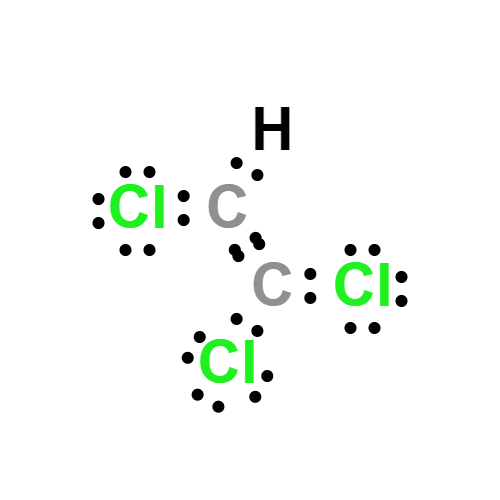
Lewis structure
- Molecular Weight:
- 131.39
- MDL Number:
- MFCD00000838
- MOL File:
- 79-01-6.mol
- MSDS File:
- SDS
| Melting point | -86 °C |
|---|---|
| Boiling point | 87 °C |
| Density | 1.463 g/mL at 25 °C(lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 61 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 90°C |
| storage temp. | 2-8°C |
| solubility | Soluble in acetone, ethanol, chloroform, ether (U.S. EPA, 1985), and other organic solvents including bromoform, carbon tetrachloride, methylene chloride, trichloroethylene, and tetrachloroethylene. |
| form | Liquid |
| color | Clear colorless |
| Odor | Chloroform-like; ethereal. |
| Odor Threshold | 3.9ppm |
| Water Solubility | Slightly soluble. 0.11 g/100 mL |
| Merck | 14,9639 |
| BRN | 1736782 |
| Henry's Law Constant | 3.14 at 1.8 °C, 8.47 at 21.6 °C, 19.0 at 40.0 °C, 26.5 at 50 °C, 35.8 at 60 °C, 56.6 at 70 °C (EPICS-GC, Shimotori and Arnold, 2003) |
| Exposure limits | TLV-TWA 50 ppm (~270 mg/m3) (ACGIH), 100 ppm (MSHA and OSHA); TLV-STEL 200 ppm (ACGIH); ceiling 200 ppm (OSHA); carcinogenicity: Animal Lim ited Evidence, Human Inadequate Evidence (IARC). |
| Dielectric constant | 3.4(16℃) |
| Stability | Stable. Incompatible with oxidizing agents, aluminium, magnesium, strong bases, reducing agents. Light-sensitive. Reacts violently with many metals, ozone, potassium nitrate, potassium hydroxide, sodium hydroxide. |
| EPA Primary Drinking Water Standard | MCL:0.005,MCLG:zero |
| LogP | 2.53 at 20℃ |
| FDA 21 CFR | 173.290; 165.110; 175.105; 73.345; 73.615 |
| Substances Added to Food (formerly EAFUS) | TRICHLOROETHYLENE |
| CAS DataBase Reference | 79-01-6(CAS DataBase Reference) |
| EWG's Food Scores | 8 |
| FDA UNII | 290YE8AR51 |
| NCI Dictionary of Cancer Terms | TCE |
| ATC code | N01AB05 |
| Proposition 65 List | Trichloroethylene |
| IARC | 1 (Vol. Sup 7, 63, 106) 2014 |
| NIST Chemistry Reference | Trichloroethylene(79-01-6) |
| EPA Substance Registry System | Trichloroethylene (79-01-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H317-H319-H336-H341-H350-H412 | |||||||||
| Precautionary statements | P202-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,F | |||||||||
| Risk Statements | 45-36/38-52/53-67-68-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 53-45-61-36/37-16-7 | |||||||||
| RIDADR | UN 1710 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KX4550000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29032200 | |||||||||
| Toxicity | LD50 orally in rats: 4.92 ml/kg; LC (4 hrs) in rats: 8000 ppm (Smyth) | |||||||||
| IDLA | 1,000 ppm | |||||||||
| NFPA 704 |
|
Trichloroethylene price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 372145 | Trichloroethylene anhydrous, contains 40?ppm diisopropylamine as stabilizer, ≥99% | 79-01-6 | 100mL | $64.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 372145 | Trichloroethylene anhydrous, contains 40?ppm diisopropylamine as stabilizer, ≥99% | 79-01-6 | 1L | $237 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1601827 | Residual Solvent Class 2 - Trichloroethylene United States Pharmacopeia (USP) Reference Standard | 79-01-6 | 3X1.2ML | $164.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 372145 | Trichloroethylene anhydrous, contains 40?ppm diisopropylamine as stabilizer, ≥99% | 79-01-6 | 2L | $126 | 2023-06-20 | Buy |
| Alfa Aesar | 047281 | Karl Fischer Solvent solvent for volumetric two-component titration | 79-01-6 | 1L | $75.65 | 2024-03-01 | Buy |
Trichloroethylene Chemical Properties,Uses,Production
Description
Trichloroethylene (IUPAC), CHClCCl2, is a stable, low-boiling, colorless liquid with a chloroform-like odor. It is not corrosive to the common metals even in the presence of moisture. It is slightly soluble in water and is nonflammable. It is toxic by inhalation, with a TLV of 50 ppm and an IDLH of 1000 ppm in air. The FDA has prohibited its use in foods, drugs, and cosmetics. The four-digit UN identification number is 1710. The NFPA 704 designation is health 2, flammability 1, and reactivity 0. Its primary uses are in metal degreasing, dry cleaning, as a refrigerant and fumigant, and for drying electronic parts.
Chemical Properties
Trichloroethylene, a colorless (often dyed blue), nonflammable, noncorrosive liquid that has the “sweet” odor characteristic of some chlorinated hydrocarbons. The Odor Threshold is 25-50 ppm.
Chemical Properties
Trichloroethylene (TCE) is a clear, colorless, nonflammable (at room temperature) stable toxic liquid with chloroform-like odor (ATSDR, 2011). It is slightly soluble in water, is soluble in greases and common organic solvents, and boils at 87°C (190 F).
On contact with air, it slowly decomposes and forms phosgene, hydrogen chloride, and dichloroacetyl chloride. Trichloroethylene in contact with water becomes corrosive and forms dichloroacetic acid and hydrochloric acid. It is soluble in methanol, diethyl ether, and acetone.

Trichloroethylene is also known as trichloroethene, acetylene trichloride, 1-chloro-2,2- dichloroethylene, and ethylene trichloride, and it is also commonly abbreviated to TRI. It is a volatile, chlorinated organic hydrocarbon that is widely used for degreasing metals and as a hydrofluorocarbon (HFC-134a) intermediate (ATSDR, 2013). It is also used in adhesives, paint-stripping formulations, paints, lacquers, and varnishes. In the 1930s, TCE was introduced for use in dry cleaning, but this practice was largely discontinued in the 1950s when TCE was replaced by tetrachloroethylene (PCE). It has a number of other past uses in cosmetics, drugs, foods, and pesticides (US EPA, 2011). It is an environmental contaminant that has been detected in air, groundwater, surface waters, and soil (US EPA, 2011; NRC, 2006).
Physical properties
Clear, colorless, watery-liquid with a chloroform-like odor. Odor threshold concentrations determined in air were 21.4 ppmv (Leonardos et al., 1969) and 3.9 ppmv (Nagata and Takeuchi, 1990). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 10 and 2.6 mg/L, respectively (Alexander et al., 1982).
Uses
Trichloroethylene is used as a solvent, in drycleaning, in degreasing, and in limited use asa surgical anesthetic.
Uses
A chlorinated hydrocarbon used as a detergent or solvent for metals, oils, resins, sulfur and as gemal degreasing agent. It can cause irritant contact dermatitis, generalized exanthema, Stevens-Johnson syndrome, pustular or bullous eruption and scleroderma.
Uses
Solvent for fats, waxes, resins, oils, rubber, paints, and varnishes. Solvent for cellulose esters and ethers. Used for solvent extraction in many industries. In degreasing, in dry cleaning. In the manufacture of organic chemicals, pharmaceuticals, such as chloroacetic acid.
Definition
ChEBI: A member of the class of chloroethenes that is ethene substituted by chloro groups at positions 1, 1 and 2.
Indications
For inhalation analgesia in obstetrics and for minor surgical procedures, but only with a special inhaler. Trichloroethylene (MAC0.17) should not be used in a closedcircuit apparatus. Trichloroethylene is better tolerated than chloroform.
Production Methods
TCE has been in commercial use for almost 60 years. TCE has been used as a solvent because of its powerful ability to dissolve fats, greases, and waxes. It has been widely used in the dry cleaning industry and as a metal degreaser and in the electronic components industry where workers have been observed using it as a cleaning solvent without any protective equipment, thus allowing uncontrolled skin contact and inhalation exposures.
General Description
A clear colorless volatile liquid having a chloroform-like odor. Denser than water and is slightly soluble in water. Noncombustible. Used as a solvent, fumigant, in the manufacture of other chemicals, and for many other uses.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Trichloroethylene has been determined experimentally that mixtures of finely divided barium metal and a number of halogenated hydrocarbons possess an explosive capability. Specifically, impact sensitivity tests have shown that granular barium in contact with monofluorotrichloromethane, trichlorotrifluoroethane, carbon tetrachloride, Trichloroethylene, or tetrachloroethylene can detonate (ASESB Pot. Incid. 39. 1968; Chem. Eng. News 46(9):38. 1968). Trichloroethylene has been determined experimentally that a mixture of beryllium powder with carbon tetrachloride or with Trichloroethylene will flash or spark on heavy impact (ASESB Pot. Incid. 39. 1968). A mixture of powdered magnesium with Trichloroethylene or with carbon tetrachloride will flash or spark under heavy impact (ASESB Pot. Incid, 39. 1968).
Health Hazard
The toxic effects manifested in humansfrom inhaling trichloroethylene vapors areheadache, dizziness, drowsiness, fatigue, andvisual disturbances. A 2-hour exposure to a1000-ppm concentration affected the visualperception. Higher concentrations can pro duce narcotic effects. Heavy exposures maycause death due to respiratory failure or car diac arrest. A 4-hour exposure to 8000 ppmwas lethal to rats. Chronic exposure causedincrease in kidney and liver weights in testanimals.
The symptoms of poisoning from oralintake of trichloroethylene are nausea, vom iting, diarrhea, and gastric disturbances. Theacute oral toxicity, however, is low. Theoral LD50 value in mice is in the range2500 mg/kg. Trichloroethylene metabolizesto trichloroacetic acid, which is excreted inthe urine.
Although trichloroethylene exhibits lowtoxicity, its metabolite trichloroethanol, andoxidative degradation products phosgene,COCl2, and chlorine, can cause severe unex pected health hazards. Kawakami andassociates (1988) reported a case of Steven–Johnson syndrome in a worker in a printingfactory. In another case, fire on a stove in ametal-degreasing workplace produced phos gene and chlorine inhalation, which causeddyspnea, fever, and fatigue.
Trichloroethylene exhibited evidence ofcarcinogenicity in laboratory animals. Oraladministration produced liver tumors, whileinhalation caused lung and blood tumors inmice and rats.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating gases are produced in fire situations.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
Trichloroethylene is used widely by industry as a metal degreaser. It is especially valuable because of its cleaning properties, low flammability, and lack of a measurable flash point. Trichloroethylene also is used as a chemical process intermediate in fluorochemical and polyvinyl chloride (PVC) production. It has been used worldwide for more than 70 years. It is a colorless, volatile liquid, and is an unsaturated aliphatic halogenated hydrocarbon. In the United States, it is produced by The Dow Chemical Company and PPG Industries, Inc. In 1998, U.S. demand was about 171 million pounds (77,700 metric tons) of which about 15 million pounds (6,800 metric tons) were imported. About 84 million pounds (38,000 metric tons) were exported. The use of trichloroethylene in 1999 can be broken down into the following categories:
chemical intermediate (~54%)
metal cleaning and degreasing (-42%)
miscellaneous (~4%)
High-purity grades of trichloroethylene are used as a feedstock in the synthesis of the refrigerant hydrofluorocarbon 134a. In this process, the trichloroethylene molecule is destroyed to form the new fluorinated compound.
Trichloroethylene's advantages for metal cleaning include the ability to degrease more thoroughly and several times faster than alkaline cleaners, and its compatibility with smaller equipment that consumes less energy. Trichloroethylene is an important solvent for degreasing aluminum and for cleaning sheet and strip steel prior to galvanizing. Trichloroethylene also is used for cleaning liquid oxygen and hydrogen tanks. Commercial trichloroethylene formulations include a stabilizer system to help prevent solvent breakdown caused by contaminants, such as acids, metal chips, and fines, and exposure to oxygen, light, and heat.
Trichloroethylene is also used as a solvent in some nonflammable adhesive and aerosol formulations, and as a low temperature heat-transfer medium. Other applications of trichloroethylene include its use as a solvent in the metal processing, electronics, printing, pulp and paper, and textile industries.
Contact allergens
Trichloroethylene is a chlorinated hydrocarbon used as a detergent or solvent for metals, oils, resins, sulfur, and as general degreasing agent. It can cause irritant contact dermatitis, generalized exanthema, Stevens-Johnson- like syndrome, pustular or bullous eruption, scleroderma, as well as neurological and hepatic disorders.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and teratogenic data. Experimental poison by intravenous and subcutaneous routes. Moderately toxic experimentally by ingestion and intraperitoneal routes. Mildly toxic to humans by ingestion and inhalation. Mildly toxic experimentally by inhalation. Human systemic effects by ingestion and inhalation: eye effects, somnolence, hallucinations or distorted perceptions, gastrointestinal changes, and jaundice. Experimental reproductive effects. Human mutation data reported. An eye and severe skin irritant. Inhalation of high concentrations causes narcosis and anesthesia. A form of addiction has been observed in exposed workers. Prolonged inhalation of moderate concentrations causes headache and drowsiness. Fatalities following severe, acute exposure have been attributed to ventricular fibrdlation resulting in cardiac failure. There is damage to liver and other organs from chronic exposure. A common air contaminant. Nonflammable, but high concentrations of trichloroethylene vapor in hightemperature air can be made to burn mildly if plied with a strong flame. Though such a condition is difficult to produce, flames or arcs should not be used in closed equipment that contains any solvent residue or vapor. Reacts with alkali, epoxides, e.g., l-chloro- 2,3-epoxypropane, 1,4-butanediol mono-2,3- epoxypropylether, 1,4-butanediol di-2,3- epoxypropylether, 2,2-bis [(4(2',3'- epoxypropoxy)phenyl)propane] to form the spontaneously flammable gas dichloroacetylene. Can react violently with Al, Ba, N2O4, Li, Mg, liquid O2, O3, KOH, KNO3, Na, NaOH, Ti. Reacts with water under heat and pressure to form HCl gas. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, ALIPHATIC.
Potential Exposure
Trichloroethylene is used as a vapor degreaser of metal parts, as a solvent; and as a drug; It is also used for extracting caffeine from coffee, as a dry-cleaning agent; and as a chemical intermediate in the production of pesticides; in making waxes, gums, resins, tars, paints, varnishes, and specific chemicals; such as chloroacetic acid.
Carcinogenicity
Trichloroethylene is reasonably anticipated to be a human carcinogen based on limited evidence of carcinogenicity from studies in humans, sufficient evidence of carcinogenicity from studies in experimental animals, and information from studies on mechanisms of carcinogenesis.
Source
A known degradation product of tetrachloroethylene.
Environmental Fate
Biological. Microbial degradation of trichloroethylene via sequential dehalogenation produced
cis- and trans-1,2-dichloroethylene and vinyl chloride (Smith and Dragun, 1984). Anoxic
microcosms in sediment and water degraded trichloroethylene to 1,2-dichloroethylene and then to
vinyl chloride (Barrio-Lage et al., 1986).
Trichloroethylene in soil samples collected from Des
Moines, IA anaerobically degraded to 1,2-dichloroethylene. The production of 1,1-
dichloroethylene was not observed in this study (Kleopfer et al., 1985).
Surface Water. Estimated half-lives of trichloroethylene (3.2 μg/L) from an experimental
marine mesocosm during the spring (8–16 °C), summer (20–22 °C), and winter (3–7 °C) were 28,
13, and 15 d, respectively (Wakeham et al., 1983).
In a laboratory experiment, the volatilization
half-life of trichloroethylene in a stirred water vessel (outer dimensions 22 x 10 x 21 cm) at 23 °C
and an air flow rate of 0.20 m/sec is approximately 1.25 h. (Kl?pffer et al., 1982).
Photolytic. Under smog conditions, indirect photolysis via OH radicals yielded phosgene, dichloroacetyl
chloride, and formyl chloride (Howard, 1990).
These compounds are readily
hydrolyzed to HCl, carbon monoxide, carbon dioxide, and dichloroacetic acid (Morrison and
Boyd, 1971). Dichloroacetic acid and hydrogen chloride were reported to be aqueous
photodecomposition products (Dilling et al., 1975). Reported rate constants for the reaction of
trichloroethylene and OH radicals in the atmosphere: 1.2 x 10-12 cm3/molecule?sec at 300 K (Hendry and Kenley, 1979), 2 x 10-12 cm3/molecule?sec (Howard, 1976), 2.36 x 10-12
cm3/molecule?sec at 298 K (Atkinson, 1985), 2.86 x 10-12 cm3/molecule?sec at 296 K (Edney et al.,
1986); with NO3: 2.96 x 10-16 cm3/molecule?sec at 296 K (Atkinson et al., 1988).
Chemical/Physical. The evaporation half-life of trichloroethylene (1 mg/L) from water at 25 °C
using a shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 18.5 min
(Dilling, 1977).
Metabolic pathway
From the photooxidation reaction medium (1) of trichloroethylene, the formation of dichloroacetyl chloride, CO, phosgene, and pentachloroethane and their conversion to the final product, CO2, are identified. By the second TiO2 photocatalyst reaction (2), trichloroacetaldehyde, dichloroacetyl chloride, CO, and phosgene with the new identified intermediates oxalyl chloride, trichloroacetyl chloride, and trichloroacetic acid are observed.
Shipping
UN1710 Trichloroethylene, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Tricloroethylene undergoes decomposition in a similar way as CHCl3, giving HCl, CO, COCl2 and organic products. It reacts with KOH, NaOH and 90% H2SO4, and forms azeotropes with water, MeOH, EtOH, and acetic acid. It is purified by washing successively with 2M HCl, water and 2M K2CO3, then dried with K2CO3 and CaCl2, then fractionally distilled before use. It has also been steam distilled from 10% Ca(OH)2 slurry, most of the water being removed from the distillate by cooling to -30o to -50o and filtering off the ice through chamois skin: the trichloroethylene is then fractionally distilled at 250mm pressure and collected in a blackened container. [Carlisle & Levine Ind Eng Chem (Anal Ed) 24 1164 1932, Beilstein 1 IV 712.]
Toxicity evaluation
Extended exposure (e.g., occupational exposure) to a chlorinated
solvent like TCE typically results in signs of central
nervous system (CNS) disturbance and hepatotoxicity.
The primary target tissues for the carcinogenicity of TCE
identified in animal studies are the liver, lung, and kidney. For
each of these target tissues there is evidence that the carcinogenicity of TCE may be associated with one or more of
its metabolites: TCA and dichloroacetic acid (DCA) in the liver,
chloral in the lung, and S-(1,2-dichlorovinyl)-L-cysteine
(DCVC) in the kidney.
TCA acts as a peroxisome proliferator, and hepatic
neoplasms in mice may arise through this mechanism.
TCE metabolism via cytochrome P450 generates the
metabolites DCA and TCA, which are associated with hepatotoxicity
and liver cancer. DCA and TCA may induce hepatic
tumorigenesis by (1) modification of signaling pathways; (2)
cytotoxicity, cell death, and reparative hyperplasia; and (3)
direct DNA damage.
TCE metabolism via glutathione (GSH) conjugation
generates DCVC, which is associated with nephrotoxicity and
kidney cancer. DCVC can be both bioactivated and detoxified
by different enzymes. The enzyme that is primarily responsible
for renal bioactivation of nephrotoxic cysteine S-conjugates is
the b-lyase. The reactive thiol and subsequent species generated
from b-lyase-catalyzed metabolism of DCVC may induce renal
tumorigenesis by (1) peroxisome proliferation, (2) a-2uglobulin
nephropathy, (3) genotoxicity leading to somatic
mutation, and (4) acute cytotoxicity and necrosis leading to cell
proliferation.
Incompatibilities
Contact with strong caustics causes decomposition and the production of highly toxic and flammable dichloroacetylene. Violent reaction with chemically active metals; powders, or shavings, such as aluminum, barium, lithium, sodium, magnesium, and titanium. Violent reaction with aluminum in the presence of dilute hydrochloric acid. Thermal decomposition of trichloroethylene, due to contact with hot metal or UV radiation, forms hazardous products including chlorine gas, hydrogen chloride; and phosgene. Keep this chemical away from high temperatures, such as arc welding or cutting, unshielded resistance heating; open flames; and high intensity UV light. Slowly decomposed by light in presence of moisture, with formulation of hydrochloric acid.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. An alternative to disposal for TCE is recovery and recycling.
Trichloroethylene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15746 | 58 |
| BLiT (Hefei)Chemical Co.,Ltd | +86-551-62622640 | sales@blitchem.com | China | 291 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7613 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
Related articles
- Trichloroethylene: Properties, Production process and Uses
- Trichloroethylene is an excellent solvent that can be used as a substitute for benzene and gasoline. It can be used to dissolv....
- Mar 19,2024
View Lastest Price from Trichloroethylene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Trichloroethylene
79-01-6
|
US $1075.00-1065.00 / Barrels | 1Barrels | 99.99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2023-12-23 | Trichloroethylene
79-01-6
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-07-27 | Trichloroethylene
79-01-6
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd |
-

- Trichloroethylene
79-01-6
- US $1075.00-1065.00 / Barrels
- 99.99%
- Hebei Dangtong Import and export Co LTD
-

- Trichloroethylene
79-01-6
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Trichloroethylene
79-01-6
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd