Chloroform
- CAS No.
- 67-66-3
- Chemical Name:
- Chloroform
- Synonyms
- CHCl3;TRICHLOROMETHANE;TCM;Fisher Chemical;Chloroform 5g [67-66-3];r20;Chloroforme;Trichlormethan;METHYLIDYNE TRICHLORIDE;Residual Solvent Class 2 - Chloroform
- CBNumber:
- CB5413313
- Molecular Formula:
- CHCl3
Lewis structure
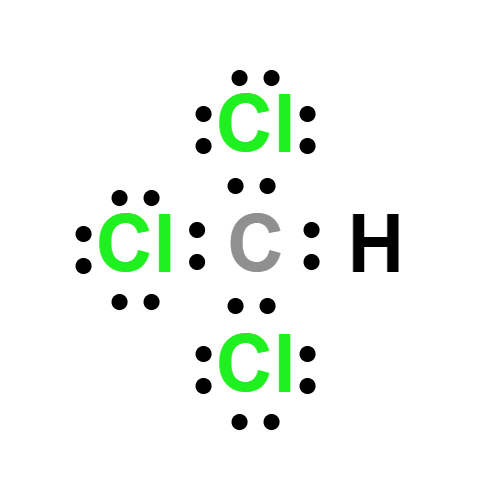
- Molecular Weight:
- 119.38
- MDL Number:
- MFCD00000826
- MOL File:
- 67-66-3.mol
| Melting point | -63 °C |
|---|---|
| Boiling point | 61 °C |
| Density | 1.492 g/mL at 25 °C(lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 160 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 60.5-61.5°C |
| storage temp. | 2-8°C |
| solubility | Miscible with diethyl ether, oils, ligroin, alcohol, carbon tetrachloride and carbon disulfide. |
| pka | 15,5(at 25℃) |
| form | Liquid |
| color | ≤10(APHA) |
| Odor | Ethereal, sweet odor detectable at 133 to 276 ppm (mean = 192 ppm) |
| Relative polarity | 0.259 |
| Odor Threshold | 3.8ppm |
| Water Solubility | 8 g/L (20 ºC) |
| λmax |
λ: 245 nm Amax: 1.00 λ: 255 nm Amax: 0.15 λ: 260 nm Amax: 0.15 λ: 270 nm Amax: 0.02 λ: 290-400 nm Amax: 0.01 |
| Merck | 14,2141 |
| BRN | 1731042 |
| Henry's Law Constant | 1.07 at 2 °C, 1.49 at 6 °C, 1.79 at 10 °C, 3.02 at 18 °C, 4.02 at 25 °C, 5.20 at 30 °C, 7.60 at 40 °C, 11.1 at 50 °C, 14.8 at 60 °C (EPICS-SPME-GC, G?rgényi et al., 2002)) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: STEL (1 h) 2 ppm (9.78 mg/m3), IDLH 500 ppm; OSHA PEL: ceiling 50 ppm (240 mg/m3); ACGIH TLV: TWA 10 ppm (adopted). |
| Dielectric constant | 5.5(0℃) |
| Stability | Volatile |
| LogP | 1.970 |
| Substances Added to Food (formerly EAFUS) | CHLOROFORM |
| FDA 21 CFR | 175.105; 177.1580; 310.545 |
| CAS DataBase Reference | 67-66-3(CAS DataBase Reference) |
| EWG's Food Scores | 7-10 |
| NCI Dictionary of Cancer Terms | TCM |
| FDA UNII | 7V31YC746X |
| ATC code | N01AB02 |
| Proposition 65 List | Chloroform |
| IARC | 2B (Vol. Sup 7, 73) 1999 |
| NIST Chemistry Reference | Chloroform(67-66-3) |
| EPA Substance Registry System | Chloroform (67-66-3) |
| Pesticides Freedom of Information Act (FOIA) | Chloroform |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H336-H351-H361d-H373 | |||||||||
| Precautionary statements | P202-P210-P233-P240-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,F,T,Xi | |||||||||
| Risk Statements | 45-46-11-23/24/25-36/37/38-48/20/22-40-38-22-67-66-36/38-41-37/38-39/23/24/25-20-63-20/22-36-48/20 | |||||||||
| Safety Statements | 9-16-26-36-36/37-45-36/37/39-25-23-53-33-7 | |||||||||
| RIDADR | UN 1888 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FS9100000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29031300 | |||||||||
| Toxicity | LD50 (14 day) orally in rats: 2.18 ml/kg (Smyth); 0.9 ml/kg (Kimura) | |||||||||
| IDLA | 500 ppm | |||||||||
| NFPA 704 |
|
Chloroform Chemical Properties,Uses,Production
Chemical Properties
At ambient temperature and pressure, chloroform exists as a clear, colorless liquid with a non-irritant ethereal odor and a sweet taste. It is very volatile, and may dissolve some plastics and rubber. It is highly water soluble and is miscible in volatile organic compounds (VOCs) and alcohols.
Uses
Chloroform (trichloromethane) is used primarily in the production of hydrochlorofluorocarbon-22 (HCFC-22) and secondarily in the production of fluoropolymers like polytetrafluoroethylene (PTFE). It has been used historically as an organic solvent, insecticidal fumigant, and as an anesthetic.

Production
- Using Acetaldehyde as raw material, react with calcium hypochlorite in alkaline solution to obtain it. Acetone can also be used as the raw material to react with calcium hypochlorite.
- Through chlorination of methane, this is a strong exothermic reaction. In order to increase the yield of chloroform and reduce the by-products (chloroform, methylene chloride, and carbon tetrachloride), the molar ratio of methane to chlorine and reacting temperature should be strictly controlled.
- Through reaction of hexachloroacetone and sodium hydroxide. CCl3COCCl3+NaOH→Chloroform+CCl3COONa
- Through reaction of carbon tetrachloride and ferrous hydroxide
- Through reaction of carbon tetrachloride and ethanol under ultraviolet irradiation CCl4+CH3CH2OH→Chloroform+ClCH2CH2OH
- Reducing carbon tetrachloride with hydrogen gas using zinc powder as a catalyst.
Reaction
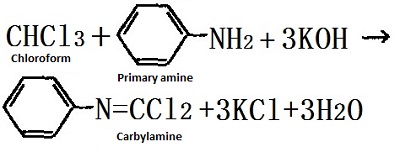
Trichloromethane is chemically active due to the induction effect of three chlorine atoms. It is easily hydrolyzed to generate formic acid and hydrogen chloride; under the alkali catalyst, addition reaction may occur, for example, chloroform and acetone undergo addition reaction at 50°C under the catalyst potassium hydroxide; in alkali solution, trichloromethane reacts with primary amine to form helium, which has a special odor to check the presence of primary amines. Trichloromethane is further chlorinated to produce carbon tetrachloride.
Trichloromethane reacts with phenol in an alkaline solution to produce hydroxyaromatic aldehydes.
Under the catalytic action of anhydrous aluminum trichloride, trichloromethane reacts with benzene to produce triphenyl(methane). This is an important method for preparing triphenylmethane dyes. 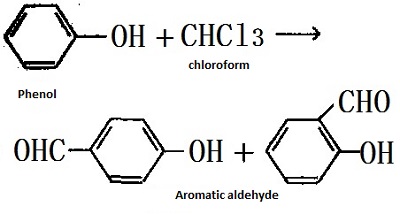
Chloroform can be oxidized in the air into highly toxic phosgene under light conditions, so chloroform is often placed in brown bottles. Commercial chloroform is also often added with 1% ethanol to destroy the small amount of phosgene generated.
Description
Chloroform is a trichlorinated organic substance with the chemical formula of CHCl3. It is a clear liquid at room temperature and has a pleasant, sweet odor. Chloroform is only slightly soluble in water and evaporates quickly into surrounding air, increasing the risk of inhalation exposure. In addition, chloroform persists for a long time in both water and air. There are no natural sources of chloroform, but this contaminant enters the environment through a variety of industrial operations, including the chlorination of water. Humans can be exposed to chloroform through inhalation, ingestion, and dermal contact.
Chloroform has a relatively narrow margin of safety and has been replaced by better inhalation anesthetics. In addition, it is believed to be toxic to the liver and kidneys and may cause liver cancer. Chloroform was once widely used as a solvent, but safety and environmental concerns have reduced this use as well. Nevertheless, chloroform has remained an important industrial chemical.
Chemical Properties
Chloroform is a noncombustible, clear, colorless liquid with a pleasant, sweet odor. The Odor Threshold is 12 ppm.
Physical properties
Chloroform is a clear, colorless liquid with a pleasant odor and sweet burning taste. It is used to make hydrochloroflurocarbons (HCFCs), as a solvent for organic chemicals, and in chemical synthesis. Its use in many commercial products has been eliminated in recent decades because of its toxic and carcinogenic properties. It was once used extensively as an anesthetic, in medicines, in dry cleaning, and in refrigerants.
History
Chloroform (CHCl3) was first discovered in 1831 by American physician Samuel Guthrie; and independently a few months later by Frenchman Eugène Soubeiran and Justus von Liebig in Germany. Chloroform was named and chemically characterised in 1834 by Jean-Baptiste Dumas. Its anaesthetic properties were noted early in 1847 by Marie-Jean-Pierre Flourens. Unlike ether, chloroform's characteristically sweet odour isn't irritating, although inhalation of concentrated chloroform vapour may cause irritation of exposed mucous surfaces. Chloroform is a more effective anaesthetic than nitrous oxide.
In 1864, the Report of Chloroform Committee of Royal Medical and Chirurgical Society endorsed chloroform as Britain's favourite anaesthetic. But ether was safer for patients.
In 1871, leading anaesthetic manufacturer Edward E. Squibb of Brooklyn estimated [New York Medical Journal (April 1871) 13;389] that of 400,000 administrations of anaesthesia in the USA in 1870, chloroform was the agent used in some 50%, ether for 40%, and other gases and mixtures accounted for the rest.
Uses
Chloroform is a widely used industrial and laboratory solvent. It is a volatile chlorinated organic solvent whose vapors have a narcotic effect.Due to its light sensitivity, it may undergo degradation with time. This can be suppressed by adding ethanol as a stabilizer. The addition will increase the polarity of the solvent and potentially impact certain applications.
- Chloroform has been used as a solvent for dissolving lipids and also as a cleansing agent.
- It may also be used in solvent extraction process and also for recrystallization.
- Suitable for HPLC, spectrophotometry, environmental testing
- Facilitates recovery of the aqueous phase of PCRs which have been overlaid with mineral oil.
- Meets ACS specifications.
Uses
In 2007, about 95% of the chloroform produced in the United States was used to make chlorodifluoromethane (HCFC-22, also known as R-22); 62% of HCFC-22 was used as a refrigerant, and 33% was used in the production of fluoropolymers (HSDB 2009). However, the use of HCFC-22 is being phased out under the 1987 Montreal Protocol, and as of January 1, 2010, manufacturers were not allowed to produce new air conditioners or heat pumps containing HCFC-22 (EPA 2010). The remaining chloroform produced has miscellaneous uses, including as a solvent or an extraction solvent for lacquers, floor polishes, adhesives in artificial silk manufacturing, resins, fats, greases, gums, waxes, oils, alkaloids, penicillin, vitamins, flavors, and rubber; as a drycleaning spot remover; in fire extinguishers; as an intermediate in the preparation of dyes and pesticides; and as a fumigant for stored grain crops (IARC 1979, ATSDR 1997, HSDB 2009). It may also be used as a local anesthetic in certain dental endodontic surgeries and in aspirin-chloroform mixtures applied topically to relieve pain from severe cases of herpes or post-therapeutic neuralgia (ATSDR 1997, HSDB 2009).
Before 1976, chloroform was used in a wide variety of drug products, including cough syrups, antihistamines, and decongestants (IARC 1979). In the 1970s, the U.S. Food and Drug Administration banned drugs containing chloroform and also banned its use in cosmetics because of its carcinogenicity. However, it did not ban drug products that contain chloroform in residual amounts resulting from its use as a solvent in manufacturing or its presence as a by-product from the synthesis of drug ingredients (IARC 1979, ATSDR 1997). An approved new drug application is required for marketing any drug product containing chloroform (FDA 1999).
Uses
Large volumes of chloroform were once used for the production of chlorofluorocarbons (CFCs), but the Montreal Protocol enacted in 1989 to eliminate CFCs as a result of their role in ozone destruction has decreased their use for this purpose. Chloroform is used to produce HCFCs, and hydrofluorocarbon (HFCs), which have been substituted for CFCs in recent years. In addition to its major use as a feedstock for HCFCs and HFCs, chloroform is used as an organic solvent in a variety of applications including pharmaceuticals, resins, lacquers, rubbers, dyes, and pesticides.Chloroform is listed as a probable human carcinogen as a result of evidence suggesting that it causes liver and kidney cancers in animals.
Production Methods
Chloroform was first synthesized by treating acetone or ethanol with calcium hypochlorite or sodium hypochlorite bleaching powder. Chlorination of ethanol produces acetaldehyde and then trichloroacetaldehyde. Acetaldehyde yields chloroform and the formate ion by action of hydroxide ion. Acetone is chlorinated to trichloroacetone, which then splits into chloroform and the acetateion. The modern industrial preparation of chloroform involves the chlorination of methane or methyl chloride, CH3Cl, using heat to substitute the chlorine atoms for hydrogen. The reaction is carried out at approximately 500°C. Hydrochlorination by reacting methanol and hydrogen chloride can also be used to produce chloroform.
Definition
A colorless volatile liquid formerly used as an anesthetic. Now its main use is as a solvent and raw material for making other chlorinated compounds. Trichloromethane is made by reacting ethanal, ethanol, or propanone with chlorinated lime.
Definition
ChEBI: A one-carbon compound that is methane in which three of the hydrogens are replaced by chlorines.
brand name
Ametuss;Benafed;Benatuss;Benyphed;Broncho-rivo syrup;Chlor-histine;Codacol;Codimal dm;Co-specto;Cotrol-d;Cyprol expectrant;Dalet;Dectuss;Eludril;Expec-c;Fk-tussex;Guanor;Histalix;Kentuss;Linctuss;Mc 3;Muflin;Nagalyn;Notose;Orthos kavident;Panosoma;Penta-zine;Phenacol-dm;Phenatuss;Phlogarol;P-m-z;Promex;Rexahisine.
World Health Organization (WHO)
Chloroform was formerly widely used in pharmaceutical preparations as a solvent and preservative as well as for its anaesthetic and flavouring properties. By the late 1970s reservations concerning its safety, including positive results in a carcinogenicity screening programme sponsored by the National Cancer Institute in the USA, had led to considerable restrictions in its use in pharmaceutical preparations. While many pharmaceutical products containing chloroform have been withdrawn or reformulated to exclude this substance, it may still be incorporated in toothpastes and other specified products in some countries, subject to statutorily-imposed concentration limits. (Reference: (IARCCD) Chloroform: IARC Monograph, 20(20), 401-427, 1979)
General Description
A clear colorless liquid with a characteristic odor. Denser (12.3 lb / gal) than water and slightly soluble in water. Hence sinks in water. Nonflammable under most conditions, but burns under extreme conditions. May cause illness by inhalation, skin absorption or ingestion. Used as a solvent, to make other chemicals, as a fumigant.
Air & Water Reactions
Slightly soluble in water. Dissolves in water to form a corrosive solution of hypochlorous acid which decomposes on standing to chlorine, oxygen, and chloric acid.
Reactivity Profile
A mixture of acetone and Chloroform in a residue bottle exploded. Since addition of acetone to Chloroform in the presence of base will result in a highly exothermic reaction, Chloroform is thought that a base was in the bottle. [MCA Case History 1661(1970)]. Powdered aluminum and carbon tetrachloride(also methyl chloride and Chloroform or mixtures of these chemicals) exploded when heated(to 153°C.) and by impact, [Chem. Eng. News 32:258(1954); UL Bull. Research 34(1945), ASESB Pot. Incid. 39(1968)]. An inadequately cooled addition of sodium to a Chloroform-methanol mixture (sodium methoxide) caused a violent explosion, [MCA Case History No. 693]. Chloroform is incompatible with dinitrogen tetraoxide, fluorine, sodium metal and alcohols, nitromethane, and triisopropylphosphine.
Hazard
A possible carcinogen. Toxic by inhalation; anesthetic; prolonged inhalation or ingestion may be fatal. It has been prohibited by FDA from use in drugs, cosmetics, and food packaging, including cough medicines, toothpastes, etc. Nonflammable. Will burn on prolonged exposure to flame or high temperature. Liver and embryo/fetal damage, and central nervous system impairment.
Health Hazard
The acute toxicity of chloroform is low by all routes of exposure. Inhalation can cause dizziness, headache, drowsiness, and nausea, and at higher concentrations, disorientation, delirium, and unconsciousness. Inhalation of high concentrations may also cause liver and kidney damage. Exposure to 25,000 ppm for 5 min can be fatal to humans. Ingestion of chloroform can cause severe burning of the mouth and throat, chest pain, and vomiting. Chloroform is irritating to the skin and eyes, and liquid splashed in the eyes can cause burning pain and reversible corneal injury. Olfactory fatigue occurs on exposure to chloroform vapor, and it is not regarded as a substance with adequate warning properties
Chloroform shows carcinogenic effects in animal studies and is listed by IARC in
Group 2B ("possible human carcinogen"). It is not classified as a "select carcinogen"
according to the criteria of the OSHA Laboratory Standard. Prolonged or repeated
exposure to this substance may result in liver and kidney injury. There is some
evidence from animal studies that chloroform is a developmental and reproductive
toxin
Health Hazard
Chloroform is classified as moderately toxic. Probable oral lethal dose for humans is 0.5 to 5 g/kg (between 1 ounce and 1 pint) for a 150 lb. person. The mean lethal dose is probably near 1 fluid ounce (44 g). It is a human suspected carcinogen. Also, it is a central nervous system depressant and a gastrointestinal irritant. It has caused rapid death attributable to cardiac arrest and delayed death from liver and kidney damage.
Fire Hazard
Container may explode in the heat of fire. When heated Chloroform liberates phosgene, hydrogen chloride, chlorine and toxic and corrosive oxides of carbon and chlorine. Chloroform explodes when in contact with aluminum powder or magnesium powder or with alkali metals (e.g., lithium, sodium, and potassium) and dinitrogen tetroxide. Chloroform reacts vigorously with acetone in the presence of potassium hydroxide or calcium hydroxide. Chloroform is oxidized by strong oxidizers such as chromic acid forming phosgene and chlorine. Chloroform reacts vigorously with triisopropylphosphine. Chloroform develops acidity from prolonged exposure to air and light.
Flammability and Explosibility
Chloroform is noncombustible. Exposure to fire or high temperatures may lead to formation of phosgene, a highly toxic gas.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
Chloroform (CHC13) is the name given to trichloromethane, CHC13, because of its supposed relation to formic acid. A colorless liquid, half as dense as water and of about the same viscosity, chloroform has a heavy, ether-like odor and a burning sweetness of taste, being about 40 times as sweet as cane sugar. It is almost insoluble in water, but it is freely miscible with organic solvents and is an important solvent for gums, resins, fats, elements such as sulfur and iodine, and a wide variety of organic compounds. Common synonyms are trichloromethane, trichloroform, freon 20, Cobehn Spray-Cleaner solvent, formyl trichloride, methane trichloride, methenyl trichloride, and methyl trichloride. Chloroform is nonflammable and does not form explosive mixtures at atmospheric temperatures and pressures. It is used primarily in the production of Chlorofluorocarbon (CFC-22) and plastics like vinyl chloride. Other uses include extraction and purification of some antibiotics, alkaloids, vitamins, and flavors. It has been used as a solvent in lacquers, floor polishes, artificial silk manufacture, resins, fats, greases, gums, waxes, adhesives, oils, and rubber; used as a solvent in photography and dry cleaning; used in fire extinguishers; and used in the preparation of dyes and pesticides. It also was once used as a general anesthetic in surgery but has been replaced by less toxic, safer anesthetics, such as ether. It now has limited use.
Chloroform evaporates quickly and in its concentrated gaseous form, it will tend to settle to the ground before dispersing. It produces poisonous gas in a fire and is unstable when exposed to air, light, and/or heat, which cause it to break down to phosgene, hydrochloric acid, and chlorine. When heated to decomposition, chloroform emits toxic fumes of hydrochloric acid and other chlorinated compounds. Chloroform is produced by reaction of chlorine with ethanol and by the reduction of carbon tetrachloride with moist iron.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. A human poison by ingestion and inhalation. An experimental poison by ingestion and intravenous routes. Moderately toxic experimentally by intraperitoneal and subcutaneous routes. Human systemic effects by inhalation: hallucinations and distorted perceptions, nausea, vomiting, and other unspecified gastrointestinal effects. Human mutation data reported. Experimental teratogenic and reproductive effects.Inhalation of the concentrated vapor causes dilation of the pupils with reduced reaction to light, as well as reduced intraocular pressure (experimental). In the initial stages there is a feeling of warmth of the face and body, then an irritation of the mucous membranes, conjunctiva, and sktn; followed by excitation, loss of reflexes, sensation, and consciousness. Prolonged inhalation will bring on paralysis accompanied by carcbac-respiratory failure and finally death. Chloroform has been widely used as an anesthetic. However, due to its toxic effects, this use is being abandoned. Concentrations of 68,000-82,000 pprn in air can kill most animals in a few minutes. 14,000 pprn may cause death after an exposure of from 30 to 60 minutes. 5000-6000 pprn can be tolerated by animals for 1 hour without serious cbsturbances. The maximum concentration tolerated for several hours or for prolonged exposure with slight symptoms is 2000-2500 ppm. Prolonged administration as an anesthetic may lead to such serious effects as profound toxemia and damage to the liver, heart, and kidneys. Experimental prolonged but light anesthesia in dogs produces a typical hepatitis. . Explosive reaction with socbum + methanol or sodium methoxide + methanol. Mixtures with sodum or potassium are impact-sensitive explosives. Reacts violently . with acetone + alkali (e.g., sodium hydroxide, potassium hydroxide, or calcium , , hydroxide), Al, disilane, Li, Mg, methanol + alkali, nitrogen tetroxide, perchloric acid + phosphorus pentoxide, potassium-tertbutoxide, sodium methylate, NaK. Incompatible with dinitrogen tetraoxide, fluorine, metals, or triisopropylphosphine. Nonflammable. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS , ALIPHATIC
Potential Exposure
Chloroform was one of the earliest general anesthetics, but its use for this purpose has been abandoned because of toxic effects. Chloroform is widelyused as a solvent (especially in the lacquer industry); in the extraction and purification of penicillin and other pharmaceuticals; in the manufacture of artificial silk, propellents, plastics, floor polishes, and fluorocarbons (R-22); and in sterilization of catgut. Chemists and support workers as well as hospital workers are believed to be at a higher risk than the general population. Chloroform is widely distributed in the atmosphere and water (including municipal drinking water primarily as a consequence of chlorination). A survey of 80 American cities by EPA found chloroform in every water system in levels ranging from ,0.3 to 311 ppb.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended.
Carcinogenicity
Chloroform is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Biological. An anaerobic species of Clostridium biodegraded chloroform (a metabolite of
carbon tetrachloride) by reductive dechlorination yielding methylene chloride and unidentified
products (G?lli and McCarty, 1989). Chloroform showed significant degradation with gradual
adaptation in a static-culture flask-screening test (settled domestic wastewater inoculum)
conducted at 25 °C. At concentrations of 5 and 10 mg/L, complete degradation was observed at
the end of the third subculture period (28 d). The amount lost due to volatilization after 10 d was
6–24% (Tabak et al., 1981).
Photolytic. Complete mineralization was reported when distilled deionized water containing
chloroform (118 ppm) and 0.1 wt % titanium dioxide as a catalyst was irradiated with UV light.
Mineralization products included carbon dioxide and HCl (Pruden and Ollis, 1983).
Photocatalyzed mineralization of chloroform in the presence of titanium dioxide as catalyst occurred at a rate of 4.4 ppm/min/gm catalyst (Ollis, 1985). Titanium dioxide suspended in an
aqueous solution and irradiated with UV light (λ = 365 nm) converted chloroform to carbon
dioxide at a significant rate. Intermediate compounds were not identified (Matthews, 1986).
Chemical/Physical. Matheson and Tratnyek (1994) studied the reaction of fine-grained iron
metal in an anaerobic aqueous solution (15 °C) containing chloroform (107 μM). Initially,
chloroform underwent rapid dehydrochlorination forming methylene chloride and chloride ions.
As the concentration of methylene chloride increased, the rate of reaction appeared to decrease.
After 140 h, no additional products were identified. The authors reported that reductive
dehalogenation of chloroform and other chlorinated hydrocarbons used in this study appears to
take place in conjunction with the oxidative dissolution or corrosion of the iron metal through a
diffusion-limited surface reaction.
storage
In the presence of light, chloroform undergoes autoxidation to generate phosgene; this can be minimized by storing this substance in the dark under nitrogen. Commercial samples of chloroform frequently contain 0.5 to 1% ethanol as a stabilizer.
Shipping
UN1888 Chloroform, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
It reacts slowly with oxygen, or oxidising agents, when exposed to air and light, giving, mainly, phosgene, Cl2 and HCl. Commercial CHCl3 is usually stabilized with up to 1% EtOH or of dimethylaminoazobenzene. Simplest purifications involve washing with water to remove the EtOH, drying with K2CO3 or CaCl2, refluxing with P2O5, CaCl2, CaSO4 or Na2SO4, and distilling. It must not be dried with sodium. The distilled CHCl3 should be stored in the dark to avoid photochemical formation of phosgene. In an alternative purification, CHCl3 (500mL) was shaken (mechanically) with several small portions of 12% H2SO4 for 1hour, washed thoroughly with water, saturated NaHCO3, washed again with water, and dried over CaCl2 or K2CO3 (100g) for 1hour before filtering and distilling. After further drying for a short time over P2O5, the CHCl3 was redistilled and stored over Drierite in the dark [Reynolds & Evans J Am Chem Soc 60 2559 1938]. EtOH can be removed from CHCl3 by passage through a column of activated alumina, or through a column of silica gel 4-ft long by 1.75-in diameter at a flow rate of 3mL/minute. (The alumina column, which can hold about 8% of its weight of EtOH, is regenerated by air drying and then heating at 600o for 6hours. It is pre-purified by washing with CHCl3, then EtOH, leaving in conc H2SO4 for about 8hours, washing with water until the washings are neutral, then air drying, followed by activation at 600o for 6hours. Just before use it is reheated for 2hour at 154o.) [McLaughlin et al. Anal Chem 30 1517 1958.] Carbonyl-containing impurities can be removed from CHCl3 by percolation through a Celite column impregnated with 2,4-dinitrophenylhydrazine (DHNP), phosphoric acid and water. (Prepared by dissolving 0.5g DHNP in 6mL of 85% H3PO4 by grinding together, then mixing with 4mL of distilled water and 10g of Celite.) [Schwartz & Parks Anal Chem 33 1396 1961]. Chloroform can be dried by distillation from powdered type 4A Linde molecular sieves. For use as a solvent in IR spectroscopy, chloroform is washed with water (to remove EtOH), then dried for several hours over anhydrous CaCl2 and fractionally distilled. This treatment removes material absorbing near 1600 cm-1 . (Percolation through activated alumina increases this absorbing impurity). [Goodspeed & Millson Chem Ind (London) 1594 1967, Beilstein 1 IV 42.] Rapid purification: Pass through a column of basic alumina (Grade I, 10g/mL of CHCl3), and either dry by standing over 4A molecular sieves, or alternatively, distil from P2O5 (3% w/v). Store away from light (to avoid formation of phosgene) and use as soon as possible.
Toxicity evaluation
Chloroform in soil or surface water volatilizes readily; at
equilibrium, greater than 99% is expected to partition to the
atmosphere. Some wet deposition of atmospheric chloroform
may occur, but subsequent revolatilization is likely to be
extensive. Chloroform is not expected to partition significantly
to soils or sediments, because its affinity for organic carbon and
lipids is low. Compartmental partitioning has been reported to
be 99.1, 0.9, 0.01, and 0.01% in air, water, soil, and sediment,
respectively. The preferred target compartment in the environment
at equilibrium is the air compartment.
Chloroform is considered as nonbiodegradable in water.
Hydrolysis is an unimportant fate process at a neutral pH value and direct photolysis in water is not expected too. In surface
water, the principal removal process is volatilization with
estimated half-lives of 1.5 days and 9–10 days in a river and
a lake, respectively. Most studies have indicated little biodegradation
up to 25 weeks in aquatic systems under aerobic
conditions. The principal fate of chloroform at the soil surface
is temperature-dependent volatilization due to its volatile
nature and low soil adsorption.
Incompatibilities
Though nonflammable, chloroform decomposes to form hydrogen chloride, phosgene, and chlorine upon contact with a flame. Chloroform decomposes slowly in air and light. Reacts violently with strong caustics (bases), strong oxidants, chemically active metals (especially powders), such as aluminum, lithium, magnesium, potassium, and sodium, causing fire and explosion hazard. Attacks plastic, rubber, and coatings. Corrodes iron and other metals in the presence of moisture.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Where possible it should be recovered, purified by distillation, and returned to the supplier.
Chloroform Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8