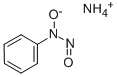
Cupferron
- CAS No.
- 135-20-6
- Chemical Name:
- Cupferron
- Synonyms
- NPHA;N-NITROSO-N-PHENYLHYDROXYLAMINE AMMONIUM SALT;Cupferon;Kupferron;CUPFERRON,REAGENT,ACS;N-Nitrosophenylhydroxylamine;N-NITROSO-N-PHENYLHYDROXYLAMINE;Kupferon;CUPFERRON;COPPERONE
- CBNumber:
- CB5679513
- Molecular Formula:
- C6H9N3O2
- Molecular Weight:
- 155.15
- MDL Number:
- MFCD00078422
- MOL File:
- 135-20-6.mol
- MSDS File:
- SDS
| Melting point | 150-155 °C (dec.) (lit.) |
|---|---|
| Boiling point | 278.95°C (rough estimate) |
| Density | 1.3092 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | 2-8°C |
| solubility | well soluble in water and ethanol. |
| form | Powder |
| color | Needles from water |
| Water Solubility | <0.1 g/100 mL at 18.5 ºC |
| Merck | 14,2622 |
| BRN | 3919107 |
| Stability | Stable, but may be moisture sensitive. Incompatible with strong oxidizing agents, strong bases, strong acids. |
| InChIKey | GDEBSAWXIHEMNF-UHFFFAOYSA-O |
| CAS DataBase Reference | 135-20-6(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | V66QK963ED |
| Proposition 65 List | Cupferron |
| NIST Chemistry Reference | Cupferron(135-20-6) |
| IARC | 2B (Vol. 127) |
| EPA Substance Registry System | Cupferron (135-20-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H319-H335-H341-H350 | |||||||||
| Precautionary statements | P201-P202-P261-P264-P301+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-36/37/38-40-45-43-23/24/25 | |||||||||
| Safety Statements | 26-36/37-45-53 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NC4725000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29299090 | |||||||||
| Toxicity | TDLo orl-rat: 123 g/kg/78W-C:CAR NCITR* NCI-CGTR-100,78 | |||||||||
| NFPA 704 |
|
Cupferron price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 675636 | Cupferron 97%, reagent grade | 135-20-6 | 100g | $297 | 2024-03-01 | Buy |
| Sigma-Aldrich | 675636 | Cupferron 97%, reagent grade | 135-20-6 | 500g | $977 | 2024-03-01 | Buy |
| TCI Chemical | C0646 | Cupferron >98.0%(HPLC)(T) | 135-20-6 | 25g | $40 | 2024-03-01 | Buy |
| TCI Chemical | C0646 | Cupferron >98.0%(HPLC)(T) | 135-20-6 | 500g | $336 | 2024-03-01 | Buy |
| Alfa Aesar | A16551 | Cupferron, 97+% | 135-20-6 | 25g | $32.65 | 2024-03-01 | Buy |
Cupferron Chemical Properties,Uses,Production
Analysis reagents
Cupferron is an important analytical reagent, as Ammonium salt of N-nitroso hydroxylamine, commonly known as cupferron. It is usually white or light yellow bright flake scrystal, with sweet odour, long home to darken due to slow decomposition, but in fact can also be used in the analysis. It is soluble in water, benzene, alcohol, ether. The reagent is thermally decomposed to prepare nitrobenzene. Unstable in light and air, we need to save itin a brown reagent grinding mouth bottle, usually place a small amount of ammonium carbonate in bottle packaged with paper or cloth good as a preservative, store in a sealed dark cool place. It can be used as a precipitating agent and solvent extraction agent of copper, iron, tin, titanium, vanadium, chromium and other elements, as a masking agent for the determination of rare earth.
Nitrobenzene is reduced with zinc powder in an aqueous solution of ammonium chloride to get phenylhydroxylamine, which is then dissolved in ether, lead to excessive ammonia, and addn-butyl nitrite, then get cupferron, can form a stable five-membered ring chelate with metal ions, therefore, it is common organic precipitant. It can quantitatively measure Fe3 + ions in the acid solution, can also be measure Cu2 +, Sn4 +, Ti4 +, Ga3 +, Hf4 +and other ions, and vanadate (VO-3, VO3-3). When Fe3 +, Cu2 + ions mixedwith other metal ions together, the reagent can be used to separate them out. It is a chelating extractant for Al3 +, Au3 +, Be2 +, Co2 +, La3 +, Pu4 + and other ions, a masking agent for rare earth metal ions.
The above information is edited by the chemicalbook of Yan Yanyong.
Purification method
Ddd 30g powdered crude into 120ml60 ℃ water, make it all dissolved, add 2g of powdered activated carbon and stir for 10~15min, filtrate with the calcined Buchner, and cool the filtrate to 10~15 ℃, then cool to 0 ℃ overnight. Suction filtration by using a glass filter to precipitate crystals, wash with 10ml ethanol and then wash with 10ml of ether, and dry in air.
Cupferron can also be recrystallized with ethanol to purify.
Chemical properties
It is white or cream colored flaky crystals. Melting point is 163-164 ℃, it is soluble in water and alcohol.
Uses
1. Cupferron is used for quantitative analysis for aluminum, zinc, copper, iron, gallium, mercury, manganese, niobium, tin, tantalum, thorium, titanium, vanadium, zirconium and other elements
2. It is used as polymerization inhibitor, due to unique polymerization inhibition characteristics of cupferron, also the amount is small, can be used as an alternative of phenol polymerization inhibitor BHT, which is current largely applicated.
3. Cupferron is used for colorimetric determination for the weight of aluminum, bismuth, copper, iron, mercury, zinc, manganese, niobium, gallium, tantalum, thorium, titanium, vanadium, tin and other elements of. It is used for quantitative determination of iron in the strong acid solution, quantitative analysis of vanadate, separation of copper and iron together with the other metals, as a masking agent for measuring rare earths.
Production method
Benzene hydroxylamine ether solution was cooled in an ice bath, lead to an excess of ammonia, while stirring and continue adding the ammonia, solution of n-butyl nitrite was added for about 1h, then stir for 15min. Filter precipitate of cupferron, wash with diethyl ether, dry to get products. The product should be stored in a brown glass bottle, the bottle is placed a small amount of ammonium carbonate wrapped by paper (or cloth) as a stabilizer.
Category
Pesticide
Toxicity grading
highly toxic
Acute toxicity
oral-rat LD 50: 199 mg/kg, intravenous-Mouse LD50: 180 mg/kg
Irritation data
Eye-rabbit 20 mg/24 hours moderate
Explosive hazardous properties
Explosive with thorium salt, zirconium salt or titanium salt solution at room temperature
Flammability hazard characteristics
It produces toxic fumes of nitrogen oxides and ammonia at high temperature.
Storage characteristics
Treasury ventilation, low-temperature drying
Extinguishing agent
Dry powder, foam, sand, carbon dioxide, water mist
Description
Cupferron is a creamy-white crystalline compound. Molecular weight=156.21; Freezing/Melting point=163℃. Hazard Identification (based on NFPA-704M Rating System): Health 2, Flammability 2, Reactivity 1.Soluble in water
Chemical Properties
white to light yellow crystalline powder
Chemical Properties
Cupferron is a creamy-white crystalline compound
Uses
Cupferron is a reagent for determination of Ce, Cu, Fe, Sn, and Ti.
Uses
The reagent used to be considered as specific for iron(II) and copper(II). However,
in the course of later analytical studies it turned out that cupferron forms
complexes with several other metals too, even in acidic medium. It is used today
primarily for the determination of copper(II), but it has proved suitable for the
determination of aluminium(III), bismuth(III), iron(III), mercury(II), thorium(IV),
tin(IV), titanium(III), vanadium(IV) and zirconium(IV) as well. Of these, the
zirconium(IV) complex is the most stable. Cupferron precipitates zirconium(IV)
quantitatively from aqueous media containing sulfuric acid.
The reagent gives a precipitate with most of the above metal ions even in strong
mineral acidic medium. Only the aluminium(III) complex does not precipitate in
the presence of mineral acids.
By means of cupferron many metal ions can be separated: thus, for instance,
iron(III) can be separated from aluminium(III) and manganese(II) and titanium
(IV), zirconium(IV) and hafnium(IV) from many other metal ions, etc. Though
the reagent makes possible several separations, the precipitates are not exactly of
stoichiometric composition (they almost always contain some excess of the ligand).
Hence instead of being directly weighed, the precipitates are usually ignited and
the metal oxide residues weighed.
Uses
As a reagent for separating Sn from Zn, and Cu and Fe from other metals. Ppts iron quantitatively from strongly acid solution; as a quantitative reagent for vanadates with which it gives a dark-red ppt soluble in alkali solution, and for Ti with which it forms a yellow ppt; also suitable for the colorimetric estimation of Al.
General Description
Light yellow or cream-colored crystals or a brown crystalline solid. As a reagent in the separation of copper and iron.
Air & Water Reactions
Hygroscopic. Soluble in water.
Reactivity Profile
Cupferron may be sensitive to prolonged exposure to air. Incompatible with strong oxidizing agents, strong acids and strong bases. Forms unstable solutions with thorium, titanium and zirconium salts.
Fire Hazard
Flash point data for Cupferron are not available; however, Cupferron is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by intravenous route. An eye irritant. Solutions with thorium salts are unstable explosives above 15°C. Solutions with titanium or zirconium salts are unstable explosives above 40℃. When heated to decomposition it emits very toxic NH3 and NOx. See also N-NITROSO COMPOUNDS and AMINES
Potential Exposure
Cupferron is used to separate tin from zinc, and copper and iron from other metals in the laboratory. Cupferron also finds application as a quantitative reagent for vanadates and titanium; and for the colorimetric determination of aluminum. The potential for exposure appears to be greatest for those engaged in analytical or research studies involving use of the chemical. Workers may also be exposed to the compound during manufacturing processes.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
Cupferron is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
storage
Color Code—Green: General storage may be used.Color Code—Blue: Health Hazard/Poison: Store in a securepoison location. Prior to working with cupferron you shouldbe trained on its proper handling and storage. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045. Cupferron should be stored in a refrigerator or in a cool dry place and protected from exposure tomoisture.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Recrystallise it twice from EtOH after treatment with Norite and finally once with EtOH. The crystals are washed with diethyl ether and air dried, then stored in the dark over solid ammonium carbonate. A standard solution (ca 0.05M prepared in air-free H2O) is prepared daily from this material for analytical work and is essentially 100% pure. [Olsen & Elving Anal Chem 26 1747 1954.] It can also be washed with Et2O, dried and stored as stated. In a sealed, dark container it can be stored for at least 12 months without deterioration. 260nm (CHCl3). max [Marvel Org Synth Coll Vol I 177 1941, Elving & Olson J Am Chem Soc 78 4206 1956, Beilstein 16 IV 891.] Possible CARCINOGEN.
Incompatibilities
Forms unstable and possibly explosive compounds with thorium salts; titanium, zirconium.
Cupferron Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
View Lastest Price from Cupferron manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-08 | Cupferron
135-20-6
|
US $30.00 / kg | 1kg | 99% | 20 tons | Anhui Ruihan Technology Co., Ltd | |
 |
2023-02-21 | Cupferron
135-20-6
|
US $60.00 / kg | 1kg | 99% | 50tons | Hebei Duling International Trade Co. LTD | |
 |
2022-10-18 | Cupferron
135-20-6
|
US $0.00-0.00 / kg | 1kg | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. |