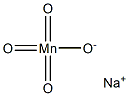
SODIUM PERMANGANATE
- CAS No.
- 10101-50-5
- Chemical Name:
- SODIUM PERMANGANATE
- Synonyms
- Natriumpermanganat;permanganatosodico;SODIUM PERMANGANATE;Sodium permangannte;permanganatedesodium;sodiumpermanganate(namno4);Permanganicacid,sodiumsalt;SodiuM perManganate, 40wt%;sodium permanganate solution;permanganatedesodium(french)
- CBNumber:
- CB5683251
- Molecular Formula:
- MnNaO4
- Molecular Weight:
- 141.925419
- MDL Number:
- MFCD00003481
- MOL File:
- 10101-50-5.mol
| Melting point | 170°C |
|---|---|
| Boiling point | 100 °C |
| Density | 1.391 g/mL at 25 °C |
| vapor pressure | 0Pa at 25℃ |
| solubility | reacts with ethanol |
| form | red-black hygroscopic crystals |
| color | red-black hygroscopic crystals, crystalline |
| Water Solubility | very soluble H2O; decomposed by alcohol [MER06] |
| Sensitive | Hygroscopic |
| Merck | 13,8727 |
| CAS DataBase Reference | 10101-50-5(CAS DataBase Reference) |
| FDA UNII | IZ5356R281 |
| EPA Substance Registry System | Sodium permanganate (10101-50-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03,GHS05,GHS07,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H272-H302-H314-H410 |
| Precautionary statements | P210-P273-P280-P301+P312+P330-P303+P361+P353-P305+P351+P338+P310 |
| Hazard Codes | O,C,N |
| Risk Statements | 8-34-50/53-22 |
| Safety Statements | 17-26-36/37/39-45-61 |
| RIDADR | UN 1503 5.1/PG 2 |
| WGK Germany | 3 |
| RTECS | SD6650000 |
| HazardClass | 5.1 |
| PackingGroup | II |
| HS Code | 28416900 |
SODIUM PERMANGANATE Chemical Properties,Uses,Production
Chemical Properties
A reddish-black or purple crystalline solid.
Chemical Properties
dark grey to purple to greenish black or black
Uses
Sodium permanganate (NaMnO4) is a purple crystal that is soluble in water and is used as an oxidizing agent, disinfectant, and bactericide and as an antidote for morphine poisoning.
Uses
Oxidizing agent; disinfectant; bactericide; man- ufacture of saccharin; antidote for poisoning by morphine, curare, and phosphorus.
General Description
A purplish colored crystalline solid. Noncombustible but accelerates the burning of combustible material. If the combustible material is finely divided the mixture may be explosive. May spontaneously ignite in contact with liquid combustible materials. Contact with sulfuric acid may cause fires or explosions. Used in medicine, as a disinfectant, and for many other uses.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Acetic acid or acetic anhydride can explode with permanganates if not kept cold [Von Schwartz 1918 p. 34]. Explosions can occur when permanganates that have been treated with sulfuric acid come in contact with benzene, carbon disulfide, diethyl ether, ethyl alcohol, petroleum, or organic matter.
Hazard
Dangerous fire risk in contact with organic materials, strong oxidizing agent.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Not classified
Safety Profile
Probably a severe irritant to the skin, eyes, and mucous membranes. A powerful oxidizer and fire hazard. Explosive reaction with acetic acid, acetic anhydride. Reacts vigorously with combustibles. When heated to decomposition it emits toxic fumes of Na2O. See also MANGANESE COMPOUNDS, PERMANGANATES, and POTASSIUM PERMANGANATE.
Potential Exposure
Used in medicine, as a disinfectant, and for many other uses: an oxidizer in reaction for making other chemicals, etchant for electronic boards; an antidote drug for chemical poisons
Shipping
UN1503 Sodium Permanganate, Hazard Class: 5.1; Labels: 5.1-Oxidizer. PG2
Incompatibilities
Oxidizers accelerates the burning of combustible material. Some may decompose explosively when heated or involved in a fire. May ignite combustibles: wood, paper, oil, organic matter, clothing, etc. Contact with finely divided metal may by explosive. May spontaneously ignite in contact with liquid combustible materials, hydrocarbons, benzene, fuels, diethyl ether, carbon disulfide. Incompatible with acetic anhydride, acetic acid. Contact with sulfuric acid may cause fires or explosions. Heat or contamination may cause explosion.
Waste Disposal
Generators of waste (equal to or greater than 100 kg/mo) containing this contaminant, EPA hazardous waste number N450, must conform to USEPA regulations for storage, transportation, treatment and disposal of waste. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Dispose of contents and container to an approved waste disposal plant. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. All federal, state, and local environmental regulations must be observed. Do not discharge into drains or sewers.
SODIUM PERMANGANATE Preparation Products And Raw materials
10101-50-5(SODIUM PERMANGANATE)Related Search:
1of4