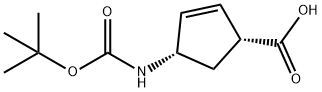
ChemicalBook >> CAS DataBase List >>(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
- CAS No.
- 151907-80-1
- Chemical Name:
- (+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
- Synonyms
- Boc-L-PEC;BOC-L-ACPEC;Peramivir impurities9;(+)-(1R,4S)-Boc-g-Homocycloleu-2-ene;(+)-(1R,4S)-Boc-g-Homocycloleu-2-ene;(+)-(1R,4S)-N-Boc-g-homocycloleu-2-ene;(+)-(1R,4S)-N-BOC-GAMMA-HOMOCYCLOLEU-2-ENE;(1R,4S)-Boc-4-aminocyclopent-2-ene-carboxylic acid;(1R,4S)-4-Boc-aMino-cyclopent-2-enecarboxylic acid;(1R,4S)-4-(Boc-amino)-2-cyclopentenecarboxylic Acid
- CBNumber:
- CB5723368
- Molecular Formula:
- C11H17NO4
- Molecular Weight:
- 227.26
- MDL Number:
- MFCD00211288
- MOL File:
- 151907-80-1.mol
- MSDS File:
- SDS
Last updated:2023-04-23 13:52:06
| Melting point | 152°C |
|---|---|
| Boiling point | 382.3±42.0 °C(Predicted) |
| Density | 1.18 |
| storage temp. | 2-8°C |
| pka | 4.31±0.40(Predicted) |
| form | Crystalline Powder |
| color | Off-white |
| optical activity | [α]20/D +48.5±2°, c = 1% in methanol |
| BRN | 6177417 |
| InChIKey | WOUNTSATDZJBLP-JGVFFNPUSA-N |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| F | 10-23 |
| HazardClass | IRRITANT |
| HS Code | 29223900 |
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | B603255 | (+)-(1R,4S)-N-Boc-4-aminocyclopent-2-enecarboxylicAcid | 151907-80-1 | 500mg | $100 | 2021-12-16 | Buy |
| TRC | B603255 | (+)-(1R,4S)-N-Boc-4-aminocyclopent-2-enecarboxylicAcid | 151907-80-1 | 100mg | $45 | 2021-12-16 | Buy |
| Matrix Scientific | 041320 | (1R,4S)-Boc-4-aminocyclopent-2-ene-carboxylic acid | 151907-80-1 | 250mg | $164 | 2021-12-16 | Buy |
| Apolloscientific | OR5860 | (1R,4S)-(-)-4-Aminocyclopent-2-ene-1-carboxylicacid,N-BOCprotected | 151907-80-1 | 5g | $267 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB44068 | (1R,4S)-4-((tert-Butoxycarbonyl)amino)cyclopent-2-enecarboxylic acid | 151907-80-1 | 1g | $285 | 2021-12-16 | Buy |
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID Preparation Products And Raw materials
Global( 147)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6711 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29321 | 58 |
| Alfa Chemistry | info@alfa-chemistry.com | United States | 2344 | 58 | |
| SpiroChem AG | contact@spirochem.com | United States | 38 | 58 |
View Lastest Price from (+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-01-06 | (+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
151907-80-1
|
US $17.00 / g | 1g | 95% | 1KG | Career Henan Chemica Co |
-

- (+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
151907-80-1
- US $17.00 / g
- 95%
- Career Henan Chemica Co
151907-80-1((+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID)Related Search:
Trans-(1S,4S)-4-Boc-aMino-2-Cyclopentene-1-carboxylic acid Methyl ester
Trans-(1R,4R)-4-Boc-aMino-2-Cyclopentene-1-carboxylic acid Methyl ester
(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid
(-)-(1S,4R)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
(-)-(1R,3S)-N-Boc-3-Aminocyclopentanecarboxylic acid
1of2
(1R,4S)-(+)-4-(BOC-AMINO)-2-CYCLOPENTENE-1-CARBOXYLIC ACID
(+)-(1R,4S)-N-T-BUTOXYCARBONYL-1-AMINOCYCLOPENT-2-ENE-4-CARBOXYLIC ACID
(1R,4S)-(-)-4-AMINOCYCLOPENT-2-ENE-1-CARBOXYLIC ACID, N-BOC PROTECTED
(1R,4S)-(-)-4-(TERT-BUTOXYCARBONYL)AMINOCYCLOPENT-2-ENE-1-CARBOXYLIC ACID
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENE-4-CARBOXYLIC ACID
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
(+)-(1R,4S)-N-BOC-GAMMA-HOMOCYCLOLEU-2-ENE
(+)-(1S,4R)-N-BOC-1-AMINOCYCLOPENT-2-ENE-4-CARBOXYLIC ACID
(1S,4R)-N-BOC-1-AMINOCYCLOPENT-2-ENE-4-CARBOXYLIC ACID
(1S,4R)-N-TERT-BUTOXYCARBONYL-1-AMINO-2-CYCLOPENTENE-4-CARBOXYLIC ACID
(1R,4S)-(+)-4-(BOC-AMINO)-2-CYCLOPENTENE -1-CARBOXYLIC ACID 98+%
(+)-(1R,4S)-N-Boc-g-homocycloleu-2-ene
(1S,4R)-N-BOC-1-Aminocyclopent-2-ene-4-carboxylic acid, 98% ee
(1R,4S)-Boc-4-aminocyclopent-2-ene-carboxylic acid
(1R,4S)-4-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylic acid
(1R,4S)-4-Boc-aMino-cyclopent-2-enecarboxylic acid
(+)-(1R,4S)-N-Butoxycarbonyl-4-aMinocyclopent-2-enecarboxylic acid
BOC-L-ACPEC
(1R,4S)-(+)-4-(Boc-aMino)-2-cyclopentene-1-carboxylic acid
Cis-(1R,4S)-4-Boc-aMino-2-Cyclopentene-1-carboxylic acid
(1R,4S)-4-[[(2-methylpropan-2-yl)oxy-oxomethyl]amino]-1-cyclopent-2-enecarboxylic acid
cis-N-tert-Butoxycarbonyl-4-aminocyclopent-2-ene-1-carboxylic acid
(+)-(1R,4S)-N-(Tert-Butoxy)Carbonyl 4-Aminocyclopent-2-enecarboxylic acid
(+)-(1R,4S)-Boc-g-Homocycloleu-2-ene
(1R,4S)-4-{[(2,2-dimethylpropanoyl)oxy]amino}cyclopent-2-ene-1-carboxylic acid
(1R,4S)-4-tert-Boc-amino-cyclopent-2-enecarboxylic acid
4S)-(+)-4-(BOC-AMINO)-2-CYCLOPENTENE-1-CARBOXYLIC ACID
4S)-N-T-BUTOXYCARBONYL-1-AMINOCYCLOPENT-2-ENE-4-CARBOXYLIC ACID
Boc-L-PEC
2-Cyclopentene-1-carboxylic acid, 4-[[(1,1-dimethylethoxy)carbonyl]amino]-, (1R,4S)-
(1S,4R)-N-BOC-1-Aminocyclopent-2-ene-4-carboxylicaci
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID USP/EP/BP
(+)-(1R,4S)-4-[(t-Butoxycarbonyl)amino]cyclopent-2-ene-1-carboxylic acid
Peramivir impurities9
(+)-(1R,4S)-Boc-g-Homocycloleu-2-ene
(+)-(1R,4S)-N-BOC-4-AMINOCYCLOPENT-2-ENECARBOXYLIC ACID
(1R,4S)-4-(Boc-amino)-2-cyclopentenecarboxylic Acid
151907-80-1
Alicyclic Amino Acids
Peptide Synthesis
Specialty Synthesis
Unnatural Amino Acid Derivatives
Unusual amino acids
Alicyclic Amino Acids
Peptide Synthesis
Unnatural Amino Acid Derivatives