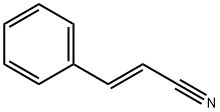
Cinnamonitrile
- CAS No.
- 1885-38-7
- Chemical Name:
- Cinnamonitrile
- Synonyms
- CINNAMALVA;Rou Guijing;naMonitrile;AKOS B004065;Ciamonitrile;Cinnamonitril;CINNAMONITRILE;STYRYL CYANIDE;CINNAMYL NITRILE;Cinnamonitrile c&t
- CBNumber:
- CB5729719
- Molecular Formula:
- C9H7N
- Molecular Weight:
- 129.16
- MDL Number:
- MFCD00001930
- MOL File:
- 1885-38-7.mol
- MSDS File:
- SDS
| Melting point | 18-20 °C (lit.) |
|---|---|
| Boiling point | 254-255 °C (lit.) |
| Density | 1.028 g/mL at 25 °C (lit.) |
| vapor pressure | 1.2Pa at 20℃ |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | soluble in Alcohol |
| form | Liquid |
| color | Clear colorless to yellow |
| Odor | at 10.00 % in dipropylene glycol. nitrile cinnamon cassia deep cumin |
| Odor Type | spicy |
| Water Solubility | insoluble |
| Sensitive | Air & Light Sensitive |
| BRN | 1209546 |
| InChIKey | ZWKNLRXFUTWSOY-QPJJXVBHSA-N |
| LogP | 1.96 at 25℃ |
| CAS DataBase Reference | 1885-38-7(CAS DataBase Reference) |
| EWG's Food Scores | 1-2 |
| FDA UNII | H475UV3WWH |
| NIST Chemistry Reference | 2-Propenenitrile, 3-phenyl-, (E)-(1885-38-7) |
| EPA Substance Registry System | 2-Propenenitrile, 3-phenyl-, (2E)- (1885-38-7) |
Cinnamonitrile price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C81004 | Cinnamonitrile 97% | 1885-38-7 | 25g | $274 | 2023-06-20 | Buy |
| Sigma-Aldrich | C81004 | Cinnamonitrile 97% | 1885-38-7 | 100g | $1040 | 2023-06-20 | Buy |
| TCI Chemical | C0361 | Cinnamonitrile >95.0%(GC) | 1885-38-7 | 25g | $59 | 2024-03-01 | Buy |
| Alfa Aesar | B25503 | Cinnamonitrile, 97%, predominantly trans | 1885-38-7 | 25g | $49.65 | 2024-03-01 | Buy |
| Alfa Aesar | B25503 | Cinnamonitrile, 97%, predominantly trans | 1885-38-7 | 100g | $129.65 | 2024-03-01 | Buy |
Cinnamonitrile Chemical Properties,Uses,Production
Flavors
Cinnamonitrile is also known as styrene cyanide, is an excellent synthetic spice,it has a strong spicy aroma like cinnamon and floral nuances, colorless or pale yellow viscous liquid, it is chemically stable, light, heat or acid, alkaline etching can not make it change. There are no toxic side effects on the human body, there is no stimulation in contact with skin ,there are no allergic reactions, compared with cinnamic aldehyde, cinnamonitrile has the stronger ability through the hair, the aroma is strong and lasts for a long time, potential skin irritation and toxicity are smaller than cinnamic aldehyde,it is the ideal substitute for cinnamon oil and cinnamic aldehyde,it can be used in daily chemical flavor formula,it is mainly used for soap flavor and fragrance detergent formulations, the amount is less than 2%. IFRA has no restrictions. Since cinnamonitrile has a unique chemical structure,it can occur Friedel, addition, reduction, hydrolysis reactions,it is also widely be used in the pharmaceutical, fine chemical industry . According to the information provided by RIFM, acute toxicity data of cinnamonitrile: oral LD504.15g/kg (rat), skin test LD50> 5g/kg (rabbit). Cinnamonitrile can be made by condensation of acetonitrile with benzaldehyde in the presence of potassium hydroxide solution ; or it can be made by the first transformation of cinnamaldehyde into oxime, then by the dehydration following.
Chemical properties
Light transparent pale yellow liquid with a strong, warm spicy fragrance.
Antibacterial effects
Nanjing University Department of Biology and the Institute of Hydrobiology of the Chinese Academy of Sciences teachers measured the inhibitory effects of plant pathogens Altornaria tenuis Nees and Rhizopus nigricans on their own separate identification , skin addiction germs Epidermophyton floccosum, Trichophytoa gypsoum, Trichophyton rubrum, Microsporum gypseum, as well as 22 kinds of mold ( 18 kinds of common food, fruits and other mold rot fungus, four kinds of human shallow filamentous fungus), the results show that cinnamonitrile has a high nitrile antimicrobial effect,it has the role of antibiotics, but it is not an antibiotic,its mold inhibition effect is good, it has broad antibacterial spectrum , and anthelmintic effect. Experiments show that the breeding of bacteria in the production process needs folic acid to participate in , cinnamonitrile structure is similar to PABA, they are competitive in binding with dihydrofolate synthase, which inhibits the activity of dihydrofolate synthase, impeding dihydrofolate synthesis, thus affecting the synthesis of folic acid, inhibiting bacterial growth propagation; and it can interfere with the synthesis of some bacteria, and the synthesis of the enzyme needed for cell division to inhibit and impact the synthesis of bacterial RNA , which reduces the permeability of the cell membrane thus affects the normal metabolism of bacteria, resulting in death, so bactericidal effect are more thorough . What is more amazing is that the human body does not produce Cinnamonitrile dependence, it can eliminate bacteria tribes, but it will not break the constraints relationship between bacteria and thus does not produce resistance. Cinnamonitrile itself is a spice, there are no toxic side effects on the human body, in contact with skin no stimulation, no allergic reactions. Experts said excitedly,that with deepening study of the cinnamonitrile antibacterial aspect , cinnamonitrile is expected to completely replace antibiotics, and become the "Terminator"of antibiotics.
Application
- Cinnamonitrile is an extremely stable nitrile spices, much like the natural aroma of cinnamon.
- For the synthesis of pharmaceuticals, perfumes and electronic chemicals.
Preparation
Cinnamic aldehyde reacts with hydroxylamine hydrochloride in the presence of pyridine by the addition-elimination reaction to produce aldehyde oxime, then it boils with toluene azeotropic and after dehydration to generate cinnamonitrile.
Chemical Properties
clear colourless to yellow liquid
Occurrence
Has apparently not been reported to occur in nature.
Uses
The parent compound whose derivatives can be applied as corrosion inhibitors for API J55 steel.
Uses
Cinnamonitrile is a parent compound whose derivatives can be applied as corrosion inhibitors for API J55 steel.
Preparation
From styryl bromide and potassium cyanide (Arctander, 1969).
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 6340, 1959 DOI: 10.1021/ja01532a069
Tetrahedron Letters, 21, p. 2161, 1980 DOI: 10.1016/S0040-4039(00)78986-6
Toxicity evaluation
The acute oral LD50 value in rats was reported as 4.15g/kg (3.67-4.63 g/kg) and the acute dermal LD50 value in rabbits exceeded 5 g/kg (Moreno, 1974). In rabbits, iv injection of cinnamyl nitrile (20.3 mg/kg) caused death in 10 min. In guinea-pigs and rabbits, cinnamyl nitrile was found to produce the same effects as hydrogen cyanide, with almost equal toxicity, and rabbits given doses higher than the lethal dose were found to die almost as rapidly as those given hydrogen cyanide (Hunt, 1923).
Flammability and Explosibility
Not classified
Metabolism
The toxicity of organic cyanides appears to depend almost entirely on whether they can be metabolized in the body to the free cyanide ion, and on the rate and extent of this conversion and the rate of detoxication of cyanide to thiocyanate. In general, alkyl and arylalkyl cyanides are metabolized with the formation of hydrogen cyanide, while the nitrile group of aryl cyanides is relatively stable. The high toxicity of iv-administered cinnamyl nitrile and the rapidity of death suggest that in rabbits this compound is metabolically converted to hydrogen cyanide (Hunt, 1923; Williams, 1959).
Cinnamonitrile Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Cinnamonitrile manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-01 | Cinnamonitrile
1885-38-7
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-03-18 | Cinnamonitrile
1885-38-7
|
US $30.00 / kg | 1kg | 99% | 20 tons | Hebei Duling International Trade Co. LTD | |
 |
2021-08-14 | Cinnamonitrile
1885-38-7
|
US $10.00 / KG | 1KG | 99 | 100 mt | Shijiazhuang tongyang Import and Export Co., LTD |
-

- Cinnamonitrile
1885-38-7
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Cinnamonitrile
1885-38-7
- US $30.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Cinnamonitrile
1885-38-7
- US $10.00 / KG
- 99
- Shijiazhuang tongyang Import and Export Co., LTD
1885-38-7(Cinnamonitrile)Related Search:
1of4