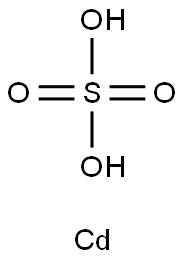
Cadmium sulfate
- CAS No.
- 10124-36-4
- Chemical Name:
- Cadmium sulfate
- Synonyms
- ACS, 99 %;CADMIUM SULFATE;CADMIUM SULPHATE;CadMiniuM Sulphate;CadmiumSulphateAcs;cadmiummonosulfate;CADMIUMSULFATE(II);CADMIUM(II)SULPHATE;cadmiumsulfate(1:1);Cadmium sulfate, p.a.
- CBNumber:
- CB5852602
- Molecular Formula:
- CdO4S
- Molecular Weight:
- 208.47
- MDL Number:
- MFCD00010923
- MOL File:
- 10124-36-4.mol
- MSDS File:
- SDS
| Melting point | 41 °C |
|---|---|
| Density | 4.691 |
| vapor pressure | 26.8hPa at 25℃ |
| solubility | water: soluble |
| form | Powder |
| color | White |
| Water Solubility | 1130 G/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,1627 |
| Exposure limits |
ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3 NIOSH: IDLH 9 mg/m3 |
| Stability | Stable. |
| CAS DataBase Reference | 10124-36-4(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | 947UNF3Z6O |
| EPA Substance Registry System | Cadmium sulfate (10124-36-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H330-H340-H350-H360FD-H372-H410 | |||||||||
| Precautionary statements | P202-P260-P264-P270-P273-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 45-46-60-61-25-26-48/23/25-50/53 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 2570 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EV2700000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28332990 | |||||||||
| NFPA 704 |
|
Cadmium sulfate price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 383082 | Cadmium sulfate ACS reagent, ≥99.0% | 10124-36-4 | 100g | $137 | 2024-03-01 | Buy |
| Alfa Aesar | 011861 | Cadmium sulfate, anhydrous, ACS, 99+% | 10124-36-4 | 25g | $29.9 | 2023-06-20 | Buy |
| Alfa Aesar | 011861 | Cadmium sulfate, anhydrous, ACS, 99+% | 10124-36-4 | 100g | $93.3 | 2023-06-20 | Buy |
| Sigma-Aldrich | 481882 | Cadmium sulfate ≥99.99% trace metals basis | 10124-36-4 | 25g | $254 | 2024-03-01 | Buy |
| Sigma-Aldrich | 481882 | Cadmium sulfate ≥99.99% trace metals basis | 10124-36-4 | 5g | $79.2 | 2023-06-20 | Buy |
Cadmium sulfate Chemical Properties,Uses,Production
Uses
Cadmium sulfate is an inorganic compound and often used in electroplating for electronic circuits. It is also used for biocatalytic etching of semiconductor cadmium sulfide nanoparticles for optical detection of analytes. In addition, Cadmium Sulfate was used to develop a DNA-based quantum dot sensor to detect ibuprofen.
Description
Cadmium sulfate is a white to colorless, odorless, crystalline substance. Molecular weight=208.48;Freezing/Melting point=1000℃. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 0, Reactivity 0. Soluble in water;solubility=76 g/100 mL at 0℃.
Chemical Properties
Cadmiumsulfate, CdS04, is an effiorescent crystalline solid that is soluble in water. It is used as an antiseptic,in the treatment of venereal diseases and rheumatism, and to detect the presence of hydrogen sulfide.
Chemical Properties
Cadmium sulfate is a white to colorless, odorless, crystalline substance.
Physical properties
Colorless orthogonal crystal; the hydrates have monoclinic crystal system; density 4.69 g/cm3 (density of mono-, and octahydrates is 3.79 and 3.08 g/cm3, respectively); melts at 1,000°C (octahydrate decomposes at 40°C); soluble in water, insoluble in ethanol.
Uses
It is a catalyst in Marsh test fir arsenic; determination of hydrogen sulfide.
Uses
Cadmium sulfate (CdS), also called “orange cadmium,” is used to produce phosphors and fluorescent screens. It is also used as a pigment in inks and paints, to color ceramics glazes, in the manufacture of transistors in electronics, photovoltaic cells, and solar cells, and in fireworks.
Uses
Applied in the formation of novel two-dimensional Cd-SCN coordination solids with unusual and tailorable, checkerboard- or herringbone-patterned structures these structures are important steps toward technologically useful materials.1
Definition
ChEBI: Cadmium sulfate is a cadmium salt.
Preparation
Cadmium sulfate is prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid:
CdO + H2SO4 → CdSO4+ H2
CdO + H2SO4 → CdSO4 + H2O
Cd(OH)2 + H2SO4 → CdSO4+ 2H2O.
General Description
Odorless white solid. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Cadmium sulfate acts as a weakly acidic inorganic salt, which is soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
A confirmed carcinogen.
Health Hazard
Inhalation may cause dryness of throat, coughing, constriction in chest, and headache. Ingestion may cause salivation, vomiting, abdominal pains, or diarrhea. Contact with eyes causes irritation.
Health Hazard
Exposures to cadmium salts by absorption are most effi cient via the respiratory tract. The symptoms of health effects include, but are not limited to, irritation, headache, metallic taste, and/or cough. Severe exposures cause shortness of breath, chest pain, and fl u-like symptoms with weakness, fever, headache, chills, sweating, nausea and muscular pain, pulmonary edema, liver and kidney damage, and death. Prolonged exposures, even at relatively low concentrations, may result in kidney damage, anemia, pulmonary fi brosis, emphysema, perforation of the nasal septum, loss of smell, male reproductive effects, and an increased risk of cancer of the lung and of the prostate. Decrease in bone density, renal stones, and other evidence of disturbed calcium metabolism have been reported.
Fire Hazard
Special Hazards of Combustion Products: Toxic cadmium oxide fume may form in fires.
Safety Profile
Confirmed human carcinogen with experimental carcinogenic data. Poison by ingestion, subcutaneous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Mutation data reported. See also CADMIUM COMPOUNDS and SULFATES. When heated to decomposition it emits very toxic fumes of Cd and SOx.
Potential Exposure
It is used in pigments, electroplating; as a fungicide; and in synthetic and analytical chemistry. Also used in fluorescent screens; as an electrolyte. Incompatibilities: Acts as a weak inorganic acid; neutralizes bases. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, sulfur, selenium, tellurium, zinc
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for atleast 15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ?ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi?cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24 48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orCadmium sulfate 515authorized paramedic may consider administering a corticosteroid spray.Note to physician: In case of fume inhalation, treat for pulmonary edema. Give prednisone or other corticosteroidorally to reduce tissue response to fume. Positive-pressureventilation may be necessary. Treat metal fume fever withbed rest, analgesics, and antipyretics. The symptoms ofmetal fume fever may be delayed for 4-12 h followingexposure: it may last less than 36 h.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective inpoisoning from cadmium.
storage
Color Code—Blue: Health Hazard: Store in asecure poison location. Prior to working with Cadmium sulfate, you should be trained on its proper handling and storage. A regulated, marked area should be established wherethis chemical is handled, used, or stored in compliance withOSHA Standard 1910.1045. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers andmetals.
Shipping
UN2570 Cadmium compounds, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with strong oxidizers,sulfur, selenium, tellurium, zinc. Acts as a weak inorganicacid; neutralizes bases. May ignite combustible materials.
Precautions
Cadmium compounds cause more health disorders to occupational workers and persons with pre-existing skin disorders, eye problems, blood disorders, prostate problems, or impaired liver, kidney, or respiratory function. These workers are more susceptible to the effects of cadmium salts. On contact with cadmium, exposed workers should wash the skin and eyes immediately with plenty of water
Cadmium sulfate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9339 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
View Lastest Price from Cadmium sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-06-15 | Cadmium sulfate
10124-36-4
|
US $23.00 / KG | 25Kg/Drum | 99% | 1000T | Baoji Guokang Bio-Technology Co., Ltd. | |
 |
2023-02-26 | Cadmium sulfate
10124-36-4
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2021-07-13 | Cadmium sulfate
10124-36-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Cadmium sulfate
10124-36-4
- US $23.00 / KG
- 99%
- Baoji Guokang Bio-Technology Co., Ltd.
-

- Cadmium sulfate
10124-36-4
- US $10.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Cadmium sulfate
10124-36-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd