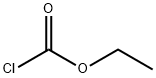
Ethyl chloroformate
- CAS No.
- 541-41-3
- Chemical Name:
- Ethyl chloroformate
- Synonyms
- ECF;CHLOROFORMIC ACID ETHYL ESTER;ETHYL CHLOROCARBONATE;LJSYZ;ethyl chlorofomate;Cathyl chloride;ETHYL CHLOROFORMIATE;ethylcarbonochloridate;Chlorine ethyl forMate;Ethyl Chloro ForMate (ECF)
- CBNumber:
- CB5853780
- Molecular Formula:
- C3H5ClO2
- Molecular Weight:
- 108.52
- MDL Number:
- MFCD00000644
- MOL File:
- 541-41-3.mol
| Melting point | -81 °C (lit.) |
|---|---|
| Boiling point | 93 °C (lit.) |
| Density | 1.135 g/mL at 25 °C (lit.) |
| vapor density | 3.74 (vs air) |
| vapor pressure | 3.42 psi ( 20 °C) |
| refractive index |
n |
| Flash point | 57 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Soluble), Ethyl Acetate (Slightly) |
| form | Liquid |
| color | White to off-white |
| Odor | Irritating; sharp, like hydrochloric acid. |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,3784 |
| BRN | 385653 |
| Dielectric constant | 11.3(20℃) |
| Stability | Moisture Sensitive |
| LogP | 1.440 (est) |
| CAS DataBase Reference | 541-41-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 09601EZP9R |
| NIST Chemistry Reference | Carbonochloridic acid, ethyl ester(541-41-3) |
| EPA Substance Registry System | Ethyl chloroformate (541-41-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H290-H302-H314-H330 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | F,T+,N | |||||||||
| Risk Statements | 11-22-26-34-50 | |||||||||
| Safety Statements | 9-16-26-28-33-36/37/39-45-61 | |||||||||
| RIDADR | UN 1182 6.1/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | LQ6125000 | |||||||||
| F | 10-19-21 | |||||||||
| Autoignition Temperature | 842 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29151300 | |||||||||
| NFPA 704 |
|
Ethyl chloroformate Chemical Properties,Uses,Production
Chemical Properties
Ethyl chloroformate is a colorless to light yellow liquid that is corrosive and flammable. It is prepared from phosgene and ethanol. It has a sharp pungent odor, like hydrochloric acid, and it decomposes in water. It is miscible with alcohol, benzene, chloroform, and ether.
Uses
Ethyl chloroformate (chloroformic acid ethyl ester) is used as a solvent in the photographic industry, and as a chemical intermediate in the production of various carbamates, and used in synthesis of dyes, drugs, veterinary medicines, herbicides, and insecticides. It is also used in the production of flotation agents for ores, as a stabilizer for PVC, and in the production of modified penicillins and heterocyclic compounds (Gerhartz, 1985).
Cathyl Chloride is used in the preparation of new inhibitors for β-homocysteine S-methyltransferase. Also used in the synthesis of a hexosaminidase inhibitor.
Preparation
Ethyl chloroformate was used in the synthesis of nitrile oxides. It can be obtained synthetically by the reaction between phosgene and anhydrous ethanol.
Ethyl chloroformate is chlorinated in the rectifying zone of a distillation reactor to produce 1-chloroethyl chloroformate and 2-chloroethyl chloroformate.
General Description
A colorless liquid with a pungent odor. Flash point 66°F. Very toxic by inhalation. Corrosive to metals and tissue. Vapors are heavier than air. Prolonged exposure to low concentrations or short exposure to high concentrations may have adverse health effects from inhalation.
Air & Water Reactions
Highly flammable. Emits fumes containing HCl on contact with moist air. Decomposes exothermically but slowly in water.
Reactivity Profile
Ethyl chloroformate decomposes slowly in water to form ethanol, HCl, and CO2 Attacks many metals especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
Inhalation causes mucous membrane irritation, coughing, and sneezing. Vapor causes severe lachrymation; liquid causes acid-type burns of eyes and skin, like those of hydrochloric acid. Ingestion causes severe burns of mouth and stomach.
Fire Hazard
Special Hazards of Combustion Products: Toxic chlorine and phosgene gases may be formed in fires.
Flammability and Explosibility
Highly flammable
Chemical Reactivity
Reactivity with Water: Slow reaction with water, evolving hydrogen chloride (hydrochloric acid); Reactivity with Common Materials: Slow evolution of hydrogen chloride from surface moisture reaction can cause slow corrosion; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. Moderately toxic by skin contact. Corrosive. An eye, skin, and mucous membrane irritant. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidzing materials. Reacts with water or steam to produce toxic and corrosive fumes. To fight fire, use CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of Cl-.
Potential Exposure
Heavily used in industry for various processes; in ore processing, photography, making other chemicals including amines, carbamates, isocyanates; polymers, diethyl carbonate; nitriles, etc.
Shipping
UN1182 Ethyl chloroformate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, 8-Corrosive material Inhalation Hazard Zone B
Purification Methods
Wash the ester several times with water, redistil it using an efficient fractionating column at atmospheric pressure and a CaCl2 guard tube to keep free from moisture [Hamilton & Sly J Am Chem Soc 47 435 1925, Saunders et al. J Am Chem Soc 73 3796 1951]. [Beilstein 3 IV 23.] LACHRYMATORY AND TOXIC.
Incompatibilities
Highly flammable; Vapors may form explosive mixture with air. Emits fumes containing HCl on contact with moist air. Decomposes exothermically but slowly in water. Ethyl chloroformate decomposes slowly in water forming ethanol, hydrogen chloride and carbon dioxide. May react vigorously, possibly explosively, if mixed with di-isopropyl ether or other ethers in the presence of trace amounts of metal salts. Reacts with acids, alkalies, amines, alcohols, oxidizers and water. Corrosive to metals especially in the presence of moisture.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices
Ethyl chloroformate Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
541-41-3(Ethyl chloroformate)Related Search:
1of4