Water
- CAS No.
- 7732-18-5
- Chemical Name:
- Water
- Synonyms
- Aqua;SODIUM HYDROXIDE SOLUTION;CAUSTIC SODA FLAKE;Di water;SODIUM HYDRATE;SODA CAUSTIC;Wasser;CAUSTIC SODA LIQUID;Oxidane;Pure Water
- CBNumber:
- CB5854297
- Molecular Formula:
- H2O
Lewis structure
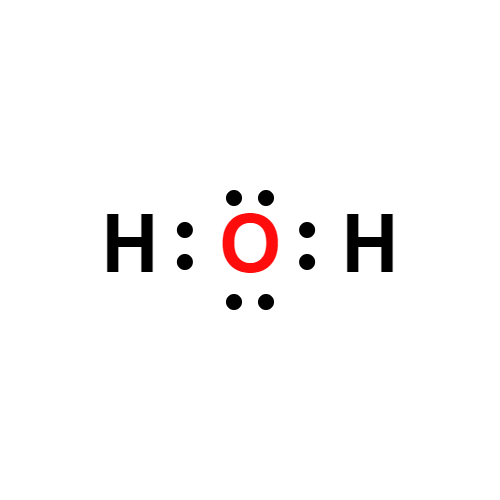
- Molecular Weight:
- 18.01
- MDL Number:
- MFCD00011332
- MOL File:
- 7732-18-5.mol
- MSDS File:
- SDS
| Melting point | 0.0 °C |
|---|---|
| Boiling point | 100 °C(lit.) |
| Density | 0.998 g/cm3 (20℃) |
| vapor density | <1 (vs air) |
| vapor pressure | 3 mm Hg ( 37 °C) |
| refractive index |
n |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Miscible in methanol, acetone, Ethanol. |
| form | liquid |
| color | colorless |
| Specific Gravity | 1.000 (20/20℃) |
| PH | 7 (20 °C) |
| Relative polarity | 9 |
| Odor | at 100.00?%. odorless |
| Water Solubility | Soluble in water. Insoluble in polar liquids. |
| λmax |
λ: 205 nm Amax: 0.01 λ: 210 nm Amax: 0.01 λ: 250-400 nm Amax: 0.005 |
| Merck | 14,10039 |
| BRN | 2050024 |
| Dielectric constant | 88.0(0℃) |
| Stability | Stable. Incompatible with reactive metals. |
| LogP | -1.380 (est) |
| FDA 21 CFR | 1230.34; 165.110; 107.10; 133.123; 133.124; 133.169; 145.110; 161.190; 201.327; 352.70; 558.258 |
| CAS DataBase Reference | 7732-18-5(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 059QF0KO0R |
| EPA Substance Registry System | Water (7732-18-5) |
| Cosmetics Info | Water |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P305+P351+P338-P321 | |||||||||
| Hazard Codes | ||||||||||
| Safety Statements | 26 | |||||||||
| RTECS | ZC0110000 | |||||||||
| F | 34 | |||||||||
| HS Code | 28530010 | |||||||||
| NFPA 704 |
|
Water price More Price(165)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | WX0004 | Water gradient grade, Meets Reagent Specifications for testing USP/NF monographs OmniSolv? | 7732-18-5 | 1L | $53.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | WX0008 | Water HPLC | 7732-18-5 | 4L | $147 | 2024-03-01 | Buy |
| Sigma-Aldrich | WX0004 | Water gradient grade, Meets Reagent Specifications for testing USP/NF monographs OmniSolv? | 7732-18-5 | 4L | $169 | 2024-03-01 | Buy |
| Sigma-Aldrich | WX0004 | Water gradient grade, Meets Reagent Specifications for testing USP/NF monographs OmniSolv? | 7732-18-5 | 20L | $742 | 2024-03-01 | Buy |
| Sigma-Aldrich | WX0003 | Water OmniTrace Ultra | 7732-18-5 | 1L | $164 | 2024-03-01 | Buy |
Water Chemical Properties,Uses,Production
Physical properties
Colorless, odorless, tasteless liquid; refractive index 1.3330; exists in three allotropic forms: solid ice, liquid water, and gaseous steam (or vapor); density of water increases with temperature, becomes maximum 1.0000 g/mL at 3.98°C and then decreases with rise in temperatures; density at 25°C 0.997 g/cm3; density of water at 100°C 0.9584 g/mL; density of steam 0.000596 g/mL at 100°C. Water freezes to ice at 0°C; expands by about 10% on freezing; boils at 100°C; vapor pressure at 0°, 20°, 50°, and 100°C are 4.6, 17.5, 92.5, and 760 torr, respectively; dielectric constant 80.2 at 20°C and 76.6 at 30°C; dipole moment in benzene at 25°C 1.76; critical temperature 373.99°C; critical pressure 217.8 atm; critical density 0.322 g/cm3; viscosity 0.01002 poise at 20°C; surface tension 73 dynes/cm at 20°C; dissolves ionic substances; miscible with mineral acids, alkalies; low molecular weight alcohols, aldehydes and ketones; forms an azeotrope with several solvents; immiscible with nonpolar solvents such as carbon tetrachloride, hexane, chloroform, benzene, toluene, and carbon disulfide.
Uses
Water is among the most important compounds on earth. It is the main constituent of the hydrosphere, which along with the mantle, crust, and the atmosphere are the four components of our planet. It is present everywhere on earth and is essential for sustenance of life. Water also determines climate, weather pattern, and energy balance on earth. It also is one of the most abundant compounds. The mass of all water on earth is 1.4x1021 kg and the total volume is about 1.4x109 km3, which includes 97.20% of salt water of oceans, 2.15% of fresh water in polar ice caps and glaciers, 0.009% in freshwater lakes, 0.008% in saline lakes, 0.62% as ground waters, 0.005% in soil moisture; 0.0001% in stream channels and 0.001% as vapors and moisture in the atmosphere. Among the major industrial applications of water are generation of hydroelectric power, steam generation, industrial solvent, diluent, moderator in nuclear reactions, industrial coolant, washing and cleaning, textile processing, preparation of food and beverages, filtration processes, and generation of hydrogen by electrolysis. Also, water provides the aqueous phase to carry out innumerable chemical reactions in the production of myriads of chemical substances including mineral acids, alkalies and their salts.
Reactions
Water undergoes autoionization to a small extent; the ionization constant at 25°C is 1.008x10–14: 2H2O(l) ↔ H3O+(aq) + OH¯ Water reacts both as an acid and a base. With bases it reacts as an acid: NH3(aq) + H2O(l) ↔ NH4+(aq) + OH¯; and with acids it reacts as a base: HCl (aq) + H2O(l) → H3O+(aq) + Cl¯(aq) Water reacts with many metal oxides and nonmetal oxides forming bases and acids, respectively: MgO(s) + H2O(l) → Mg(OH)2(s) CaO(s) + H2O(l) → Ca(OH)2 (s) N2O5(s) + H2O(l) → 2HNO3(l) P4O10(s) + 6H2O (l) → 4H3PO4(s) Water also behaves both as an oxidizing and reducing agent. With alkali and alkaline earth metals, which are strong reducing agents, water acts as an oxidizing agent. Reactions occur violently or vigorously at ambient temperatures with all alkali metals and calcium, strontium, and barium forming their hydroxides with liberation of hydrogen: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) Ca(s) + 2H2O(l) → Ca(OH)2(s) + H2(g) With less active metals, reactions occur at high temperatures. In such reactions oxides are formed instead of hydroxides, liberating hydrogen: Mg(s) + H2O(l) → MgO(s) + H2(g) Ni(s) + H2O (l) → NiO(s) + H2(g) 3Fe(s) + 4H2O (l) → Fe3O4(s) + 4H2(g) Water reacts with nonmetals and metalloid elements at very high temperatures forming oxides: C(s) + H2O(g) →CO(g) + H2(g) Ge(s) + 2H2O(l) →GeO2(s) + 2H2 (g) Water behaves as a reducing agent in reactions with oxidizing agents: 2F2 (g) + 2H2O (l) → 4HF (aq) + O2 (g) Water reacts with carbon monoxide at high temperatures (200 to 400°) in the presence of a catalyst to yield carbon dioxide and hydrogen. The reaction also is known as water-gas shift reaction: CO(g) + H2O(l) ↔ CO2 (g) + H2 (g) Water reacts with metal hydrides liberating hydrogen. With the hydrides of sodium and potassium the reaction progresses with explosive violence: NaH + H2O → NaOH + H2 With alkali metal amides violent reactions occur, forming alkali hydroxides and ammonia: NaNH2 + H2O → NaOH + NH3 Violent reactions occur with lithium aluminum hydride and similar compounds: LiAlH4 + 4H2O → LiOH + Al(OH)3 + 4H2 Sodium ethoxide decomposes in water forming sodium hydroxide and ethanol: NaOC2H5 + H2O → NaOH + C2H5OH Sulfuryl chloride, SO2Cl2, reacts with ice-cold water to form a hydrate, SO2Cl2•15H2O. However, at ambient temperature water decomposes sulfuryl chloride slowly forming sulfuric acid and hydrochloric acid: SO2Cl2 + 2H2O → H2SO4 + 2HCl Water reacts with calcium carbide to form acetylene: CaC2 + H2O → C2H2 + CaO Water forms hydrates with a large number of metal salts. Such hydrates are formed from absorption of moisture from air by anhydrous salts. Examples are Na2SO4•7H2O, CuSO4•5H2O, and BaCl2•2H2O. In many salt hydrates, water molecules coordinate to the metal ions, e.g., [Ni(H2O)6](NO3)2. Organic esters are hydrolyzed to form corresponding organic acids and alcohol. The reaction is catalyzed by acids: RCOOR’ + H2O → RCOOH + R’OH
Production Methods
Water is produced by combustion of hydrogen with oxygen at high temperatures in the presence of a catalyst. Also, all combustion reactions of hydrocarbons (C, H compounds) or oxygenated hydrocarbons (C, H, O) yield water and carbon dioxide:
CH4 + 2O2 → CO2 + 2H2O
2CH3OH + 2O2 → 2CO2 + 4H2O
All acid-base neutralization reactions form water:
HCl + NaOH → NaCl + 4H2O
Organic condensation reactions eliminate a water molecule:
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
Many hydroxides dehydrate at high temperatures forming oxides and water:
Ca(OH)2 → CaO + H2O
Water can be purified by distillation, ion exchange, filtration, carbon adsorption, and chlorination.
Chemical Properties
colourless liquid
Uses
For use in embryo manipulation.
Uses
For use in the preparation of cell culture media, and cell suspension and washing solutions.
Uses
Water is a colorless, odorless, tasteless liquid formed by the combi- nation of two hydrogen and one oxygen atoms. it allows substances to dissolve and functions as a solvent, dispersing medium, hydrate, and promoter of chemical changes. it is a major constituent in meats, fruits, and vegetables. distilled water is obtained by conden- sation of water vapor.
Uses
water is listed also as catalyzed, deionized, demineralized, distilled, pure spring, and purified water. Water is an important skin component and is essential for its proper functioning. It is the most common ingredient used in cosmetic formulations and, therefore, is generally listed first on product labels. Water is usually processed to eliminate hardness and minerals, and to avoid product contamination.
Definition
ChEBI: An oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms.
General Description
Water is widely used for HPCE (High-Performance Capillary Electrophoresis), luminescence and UV-spectroscopic studies.
Purification Methods
Conductivity water (specific conductance ca 10-7 mho) can be obtained by distilling water in a steam-heated tin-lined still, then, after adding 0.25% of solid NaOH and 0.05% of KMnO4, distilling once more from an electrically heated Barnstead-type still, taking the middle fraction into a Jena glass bottle. During these operations suitable traps must be used to protect against entry of CO2 and NH3. Water, only a little less satisfactory for conductivity measurements (but containing traces of organic material) can be obtained by passing ordinary distilled water through a mixed bed ion-exchange column containing, for example, Amberlite resins IR 120 (cation exchange) and IRA 400 (anion exchange), or Amberlite MB-1. This treatment is also a convenient one for removing traces of heavy metals. (The metals Cu, Zn, Pb, Cd and Hg can be tested for by adding pure concentrated ammonia to 10mL of sample and shaking vigorously with 1.2mL of 0.001% dithizone in CCl4. Less than 0.1Yg of metal ion will impart a faint colour to the CCl4 layer.) For almost all laboratory purposes, simple distillation yields water of adequate purity, and most of the volatile contaminants such as ammonia and CO2 are removed if the first fraction of distillate is discarded. Most laboratories have glass stills that “doubly” or “trebly” distil water. [See “water” in Chapter 1.]
Water Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Jinan Liheng Biotechnology Co., Ltd. | 15865262812 | info@lihengpharm.com | CHINA | 938 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
Related articles
- Exploring the Compound H2O: Water
- Water, the essential substance for life as we know it, is a compound with the chemical formula H2O.
- Feb 27,2024
- Does the Molecule of Water Have Ionic Charge?
- Water(H2O) molecule maintains neutrality. but it does exist in equilibrium with hydrogen cation and hydroxide anion that are r....
- Feb 5,2024
- A polar molecule-water
- water is polar because it has polar covalent bonds and is asymmetrical. A bent structure gives the water molecule its asymmetr....
- Dec 18,2023
Related Qustion
- Q:What is the pKa of water?
- A:Based on thermodynamics, the true pKa of water is 14. However, there are studies that propose a pKa of 14 or 15.74.
- Feb 29,2024
View Lastest Price from Water manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-26 | Water
7732-18-5
|
US $100.00 / kg | 1kg | 99% | 500t/month | Henan Bao Enluo International TradeCo.,LTD | |
 |
2023-03-31 | Acetyl Tetrapeptide-11
7732-18-5
|
US $0.00 / G | 1G | 99% | 100KG | Chengdu Youngshe Chemical Co., Ltd. | |
 |
2022-02-23 | Sterile water
7732-18-5
|
US $2.00-0.80 / bottle | 10bottle | 99.99%HPLC.USP42 | 1000kgs | Shanghai Biolang Biotechnology Co., Ltd. |
-

- Water
7732-18-5
- US $100.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD
-

- Acetyl Tetrapeptide-11
7732-18-5
- US $0.00 / G
- 99%
- Chengdu Youngshe Chemical Co., Ltd.
-

- Sterile water
7732-18-5
- US $2.00-0.80 / bottle
- 99.99%HPLC.USP42
- Shanghai Biolang Biotechnology Co., Ltd.
7732-18-5(Water)Related Search:
1of4





