Potassium dichromate
- CAS No.
- 7778-50-9
- Chemical Name:
- Potassium dichromate
- Synonyms
- POTASSIUM BICHROMATE;kaliumdichromat;bichromateofpotash;CHROMIUM POWDER;YEAST BOUND CHROMIUM;Potassiumdichromate,99%;ZGSJ;iopezite;CHROMIUM METAL;HTM BROTH 100ML
- CBNumber:
- CB5854318
- Molecular Formula:
- Cr2K2O7
- Molecular Weight:
- 294.1846
- MDL Number:
- MFCD00010944
- MOL File:
- 7778-50-9.mol
- MSDS File:
- SDS
| Melting point | 398 °C (lit.) |
|---|---|
| Boiling point | 82 °C |
| Density | 7.14 g/mL at 25 °C(lit.) |
| Flash point | 50 °F |
| storage temp. | Store at RT. |
| solubility | H2O: 0.1 M at 20 °C, clear, orange |
| form | powder |
| color | Orange-red |
| Specific Gravity | 2.676 |
| PH | 3.5-5.0 (25℃, 0.1M in H2O) |
| Water Solubility | 125 g/L (20 ºC) |
| Merck | 14,7627 |
| Exposure limits |
ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| InChIKey | KMUONIBRACKNSN-UHFFFAOYSA-N |
| CAS DataBase Reference | 7778-50-9(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | POTASSIUM DICHROMATE |
| FDA 21 CFR | 175.390 |
| EWG's Food Scores | 5 |
| FDA UNII | T4423S18FM |
| EPA Substance Registry System | Potassium dichromate (7778-50-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS03,GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H301-H312-H314-H317-H330-H334-H335-H340-H350-H360FD-H372-H410 | |||||||||
| Precautionary statements | P210-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T,N,T+,O | |||||||||
| Risk Statements | 45-46-60-61-8-21-25-26-34-42/43-48/23-50/53-52/53-20-48/20-23-51/53-22-36/37/38-27-20/21-23/24 | |||||||||
| Safety Statements | 53-45-60-61-36/37-23-26 | |||||||||
| RIDADR | UN 3086 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HX7680000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28415000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 90.5 mg/kg LD50 dermal Rat 1170 mg/kg | |||||||||
| NFPA 704 |
|
Potassium dichromate Chemical Properties,Uses,Production
General description
Potassium dichromate (Formula K2Cr2O7) will be decomposed and releases oxygen under the white heat temperature. It has strong oxidizing property, being a strongly toxic and carcinogenic oxidant. It appears as orange-red solid at room temperature and plate crystal. It has been classified as a Category I carcinogen by the International Agency for Research on Cancer (IARC), and may be combustible when come into contact with combustibles. At temperature above 500 ° C, it can lead to oxidation to generate chromic acid and chromium oxide. Potassium dichromate has very small solubility at low temperature and does not contain crystal water. It is easily purified through recrystallization; also not easy to have deliquescence and thus often being used as the reference standard in the analysis.
Owing to the strong oxidizing properties of potassium dichromate under acidic conditions, it is commonly used in the laboratory to formulate a chromic acid washing agent (a mixture of saturated potassium dichromate solution and concentrated sulfuric acid) for washing the chemical glassware in order to remove the reductive dirt in the glass wall. After use, the washing agent changes from dark red to green color under which the washing agent becomes invalid. Potassium dichromate can also used be in analytical chemistry, commonly used to determine the reductive hydrogen sulfide, sulfurous acid, ferrous ions and so on. Upon the heating, potassium dichromate can also oxidize concentrated hydrochloric acid to release chlorine.
As the raw materials for the production of leather hydrolysates are mainly from the corner of the tannery factory waste, and these wastes often contain potassium dichromate and sodium dichromate, during the production of hydrolyzed protein, potassium dichromate, sodium dichromate will be naturally brought into the dairy products. Human drinking can lead to joint osteoporosis, swelling and other poisoning problems. Chroming workers subjecting to repeated or long-term exposure to low concentrations of chromium compounds can get chronic upper respiratory tract inflammation, chrome rhinopathy, contact dermatitis and rash which mainly occur in naked places such as the face, neck, hands and forearm. It can also cause kidney and liver damage as well as causing blood system changes. The occurrence of lung cancer has a incubation period of 10-20 years.
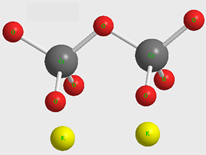
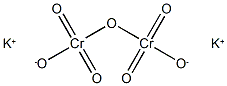
Figure 1 the molecular structure of the potassium dichromate
Defination
Potassium bichromate is an odorless substance that can be found naturally occurring in the environment, for instance, in ash, sand, loam, and clay. The chemical occurs in steel-made tools, and chrome-plated objects such as needles, silverware, handles, and bracelets. Furthermore, potassium dichromate is used as a component of fur-tanning agents, leather, dark textile dyes, epoxy hardeners, chromium pigments, and photographic color developer. The orange-red color powdered substance has a melting point of 398oC and a molecular weight of 294 gram (g)/mol. Notably, the chemical is soluble in water and ethyl alcohol.
Production
The production of potassium bichromate begins with the reaction of chromium trioxide and potassium hydroxide in a reactor generating a mother liquor.
2 CrO3 + 2 KOH = K2Cr2O7 + H2O
The next step is filtration of the mother liquor and the ensuing filter solids are put aside for disposal. The remaining substance is then sent for precipitation of crystalline potassium bichromate through a centrifugation process. Finally, the mother liquor from the centrifuge is sent back to the reactor.
Application
Potassium bichromate is an inorganic oxidizing agent that has multiple scientific and industrial uses.
Photography
Potassium bichromate has significant uses in photographic screen-printing and photography where together with strong mineral acid, they can be used as an oxidizing agent. In the 1850s, gum bichromate printing was largely used in the photographic printing processes. It is worth noting that when exposed to ultraviolet light, a solution of potassium bichromate and gum Arabic will be dried and hardened. Together with concentrated hydrochloric acid (HCL), photochromos and chromium uses potassium bichromate to treat thin and weak negatives of black and white photograph roll. The solution of HCL together with potassium bichromate reconverts the particles of silver in the film to silver chloride. The film is the exposed to actinic light and then redeveloped to its end product, giving out a more stronger negative and a acceptable print. In sulfuric acid, potassium bichromate solution can be used to manufacture a reversal negative.
Industrial Uses
Manufacturers in the textile industry use the chemical in dying clothe fabrics and tanning leather. In addition, potassium bichromate is normally used in decorative chromium plating as well as coating active metals such as steel and aluminum to prevent them from corrosion. In science, the substance is used as a solvent and in the identification of certain elements in solutions. In the furniture industry, the chemical is used in straining wood.
Health Effects
Acute Health Effects
Potassium bichromate, similar to other chromate compounds can cause acute health effects if inhaled or ingested. The chemical can cause irritation in the lungs or throats of some individuals. Chrome fumes and dust are irritating to the lungs and respiratory tract. Toxic effects due to over exposure may lead to asthmatic conditions. Accidental ingestion of the chemical may lead to toxic effects.
Potassium bichromate may result to chemical burns within the gastrointestinal tract and oral cavity if swallowed. It is noteworthy that acute poisoning from the substance is minimal since vomiting or renal excretion occurs immediately after swallowing. However, swallowing the chemical can lead to extreme thirst, loss of appetite, fever, augmented volumes of urine, gastric imbalances, and convulsions. Due to failure of breathing and inflammation of the bowel and the stomach, an individual can die following the swallowing of potassium bichromate.
Following direct contact to the eye, the chemical can produce burns and cause severe damage. If in contact with the skin and absorbed, potassium bichromate may lead to chemical burns. In addition, chrome fumes such as chrome oxide may exacerbate current skin conditions, for instance, eczema or dermatitis, and can lead to severe damage to the skin. Also, absorption through the skin may lead to poisoning affecting the liver and kidneys. It is essential to note that the substance should not come to contact with open cuts, lesions, or abrasions as it may produce systemic injuries if it enters the blood stream.
Chronic Health Effects
Potassium bichromate is a mutagen and a carcinogen, therefore can cause cancer in humans due to prolonged exposure. In addition, lengthy exposure inside the mouth can lead to ulcerative and inflammatory changes in the mouth and erosion of the teeth. Recurrent attach of bronchial pneumonia and the gastrointestinal disturbances may ensue. Inhalation and skin contact with potassium bichromate may result to sensitization reaction is particular individuals. Interestingly, the substance can lead to genetic defects as well as reduced fertility in humans.
Waste Disposal
No waste solutions of potassium chromate should be drained, as they can cause detrimental effects to the environment. In addition, all powdered materials should be disposed as hazardous waste. Generally, all disposable materials as well as decontamination from non-disposable tools or equipment must be disposed ass hazardous waste.
References
- https://nj.gov/health/eoh/rtkweb/documents/fs/1564.pdf
- http://datasheets.scbt.com/sc-203353.pdf
- http://archived.materials.drexel.edu/Safety/SOP/sop_potassium_dichromate.pdf
- Jessica Jacobs. What Is Potassium Chromate Used For? https://www.livestrong.com/article/1013080-livestrongs-future-food-chef-dinner-chef-tal-ronnen-impossible-burger/
- https://archive.epa.gov/epawaste/hazard/web/pdf/pot-dich.pdf
Chemical Properties
Also known as potassium bichromate and red potassium chromate, K2Cr2O7 is poisonous,yellowish-red crystals with a metallic taste that is soluble in water,insoluble in alcohol,that melt at 396℃;and decompose at 500℃. Used as an oxidizing agent and analytical reagent,and in explosives, matches, and electroplating.
Chemical Properties
Potassium chromate(VI) is a yellow crystalline solid.
Uses
Used (1) in matches, (2) in leather tanning and in the textile industry, (3) as a source of chromate, (4) in pyrotechnics, (5) in colored glass, (6) as an important laboratory reagent, (7) in blueprint developing, and (8) in wood preservation formulations.
Uses
Potassium bichromate is also known as potassium dichromate, this bright orange crystal was made by the acidification of potassium chromate. It is toxic and an oxidant and is soluble in water but not in alcohol. In 1839 Mungo Ponton sensitized paper with a solution of potassium bichromate to print-out images in the sun. The most important use of potassium bichromate was used to make colloids sensitive to light for a variety of processes such as the photoglyphic engraving process, the carbon process, the gum bichromate process, and in the preparation of the gelatin relief used to make lead molds for the Woodburytype.
Uses
In tanning leather, dyeing, painting, decorating porcelain, printing, photolithography, pigment-prints, staining wood, pyrotechnics, safety matches; for bleaching palm oil, wax, and sponges; waterproofing fabrics; as oxidizer in the manufacture of organic chemicals; in electric batteries; as depolarizer for dry cells. As corrosion inhibitor in preference to sodium dichromate where lower soly is advantageous. Pharmaceutic aid (oxidizing agent).
Definition
ChEBI: A potassium salt that is the dipotassium salt of dichromic acid.
General Description
Orange red crystals. Denser than water and soluble in water. No distinctive odor. May severely irritate the eyes and respiratory tract. Avoid contact with organic materials. Noncombustible. Used in pyrotehnic displays with tungsten and iron.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Potassium or sodium dichromate reacts explosively with hydrazine [Mellor 11:234. 1946-47]. A drop of anhydrous hydroxylamine on powdered potassium dichromate produces a violent explosion [Mellor 8:293. 1946-47].
Hazard
Toxic by ingestion and inhalation. Dan- gerous fire risk in contact with organic materials. Strong oxidizing agent.
Health Hazard
Highly corrosive to skin and mucous membranes. If ingested, causes violent gastroenteritis, peripheral vascular collapse, vertigo, muscle cramps, coma, and (later) toxic nephritis with glycosuria. Allergic reactions may also occur.
Fire Hazard
Behavior in Fire: May decompose, generating oxygen. Supports the combustion of other materials.
Industrial uses
This material, K2Cr2O7, decomposes at 500°C.Bright yellowish-red crystals are soluble andpoisonous. Sometimes potassium chromate,K2Cr2O4, and the dichromate are utilized inceramics as coloring agents.
Potassium dichromate is used in glass foraventurine effects. It is said that 20 or 21 partsto 100 parts sand will give a chrome aventurine.This glass is characterized by glittering metallicscales of chromium oxide. Potassiumdichromate is also used in glass to give a greencolor. However, it has been shown that it may cause considerable trouble by formation ofblack, chrome corundum crystals in the glass.Air-floated chromite is suggested to avoid thisproblem.
Potassium dichromate is used in glazes toproduce chrome-tin pinks, low-fire reds, greens,and purplish-red colors.
Safety Profile
Human poison by ingestion. An experimental poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Human mutation data reported. An experimental teratogen. Other experimental reproductive effects. Flammable by chemical reaction. A powerful oxidzer. Explosive reaction with hydrazine. Reacts violently or ignites with H2SO4+ acetone, hydroxylamine, ethylene glycol (above 100℃). Forms pyrotechnic mixtures with boron + dion, iron (igmtes at 1090℃), tungsten (igmtes at 1700℃). Reacts with sulfuric acid to form the strong oxidant chromic acid. Used in photomechanical processing, chrome pigment production, and wool preservation methods. When heated to decomposition it emits toxic fumes of K2O. See also CHROMIUM COMPOUNDS.
Potential Exposure
Potassium chromate is used in printing: photomechanical processing; chrome-pigment production; and wool preservative methods; to make dyes, pigments, inks and enamels; as an oxidizing agent; analytical reagent; in electroplating; explosives.
Shipping
UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from water (g/mL) between 100o and 0o and dry it under vacuum at 156o. (Possible CARCINOGEN.)
Incompatibilities
A powerful oxidizer. Violent reactions with combustibles, organics, powdered metals; or easily oxidizable substances. Contact with hydroxylamine, hydrazine causes explosion.
Potassium dichromate Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
7778-50-9(Potassium dichromate)Related Search:
1of4