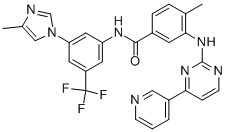
Nilotinib
- CAS No.
- 641571-10-0
- Chemical Name:
- Nilotinib
- Synonyms
- Tasigna;AMN 107;Nilotinib-d6;4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4 -pyridin-3-ylpyrimidin-2-yl)amino]benzamide;CS-307;Nilotinib;Nilotinibr;Nilotinib &aMp;AMN107; TASIGNA;Nilotinib, >=99%
- CBNumber:
- CB5966228
- Molecular Formula:
- C28H22F3N7O
- Molecular Weight:
- 529.52
- MDL Number:
- MFCD09833716
- MOL File:
- 641571-10-0.mol
| Melting point | 231-233 °C |
|---|---|
| Density | 1.36 |
| storage temp. | -20°C Freezer |
| solubility | Soluble in DMSO (up to 50 mg/ml) |
| form | Beige powder. |
| color | Off-white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| InChIKey | HHZIURLSWUIHRB-UHFFFAOYSA-N |
| SMILES | N1(C=C(C)N=C1)C1C=C(C=C(C=1)NC(=O)C1C=CC(C)=C(NC2=NC=CC(C3=CC=CN=C3)=N2)C=1)C(F)(F)F |
| CAS DataBase Reference | 641571-10-0(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Tasigna |
| FDA UNII | F41401512X |
| NCI Drug Dictionary | Tasigna |
| ATC code | L01EA03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| HS Code | 29335990 |
Nilotinib price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 10010422 | Nilotinib ≥95% | 641571-10-0 | 5mg | $25 | 2024-03-01 | Buy |
| Cayman Chemical | 10010422 | Nilotinib ≥95% | 641571-10-0 | 10mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 10010422 | Nilotinib ≥95% | 641571-10-0 | 25mg | $73 | 2024-03-01 | Buy |
| Cayman Chemical | 10010422 | Nilotinib ≥95% | 641571-10-0 | 50mg | $118 | 2024-03-01 | Buy |
| Usbiological | 460767 | Nilotinib-d6 | 641571-10-0 | 1mg | $566 | 2021-12-16 | Buy |
Nilotinib Chemical Properties,Uses,Production
Nilotinib for the treatment of chronic myeloid leukemia
Nilotinib is a novel drug for targeted cancer therapy and belongs to tyrosine kinase inhibitors for the treatment of patients of chronic myelogenous leukemia (CML) which is resistant to the Gleevec (imatinib) with an excellent efficacy. Gleevec is the primary-choice drug developed by Novartis Company for the treatment of chronic myelogenous leukemia (CML) .
Nilotinib is the developed through the improvement of the molecular structure of imatinib with a stronger selectivity on the BCR-ABL kinase activity. The inhibitory effect of nilotinib on the tyrosine kinase is 30 times as high as that of imatinib. It is capable of suppressing the activity of the imatinib-resistant BCR-ABL mutant kinase while also being able to inhibit the activity of KIT and PDGFR kinase.
With administration twice daily, nilotinib can targeted to the Bcr-Abl protein, interact with it and inhibit the emergence of cancer cells containing abnormal chromosomes. Bcr-Abl protein is produced by cells containing the abnormal Philadelphia chromosome. For patients of CML, this protein is considered to be an important factor for causing the excessive proliferation of cancer-causing white blood cells.
Approved Uses:
TASIGNA® (nilotinib) capsules is a prescription medicine used to treat:
Adults with newly diagnosed Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in chronic phase
Adults with Ph+ CML in chronic phase and accelerated phase who no longer benefit...
https://www.novartis.us
Mechanism of Action
Nilotinib is a selective tyrosine kinase inhibitor active against Bcr-Abl kinase Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL protein. Nilotinib is 30-fold more potent than imatinib.
Absorption, Fate, and Excretion
Nilotinib is rapidly absorbed and reaches its peak concentration in 3 hours. Nilotinib AUC was increased by 82% when given 30 minutes after a high-fat meal compared with a fasting state. Its elimination half-life is approximately 17 hours. It is metabolized by oxidation and hydroxylation as well as undergoing metabolism by CYP3A4. None of the nilotinib metabolites have significant pharmacologic activity.
Drug Interactions
Nilotinib is a competitive inhibitor of cytochrome P-450 (CYP) isoenzymes 3A4, 2C8, 2C9, and 2D6 and has the potential to increase concentrations of drugs metabolized by these enzymes. Nilotinib plasma concentration is increased during concomitant use with potent CYP3A4 inhibitors (e.g., atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole). Decreased nilotinib plasma concentration occurs during concomitant use with potent CYP3A4 inducers (e.g., dexamethasone, carbamazepine, phenobarbital, phenytoin, rifabutin, rifampin, and St. John's wort). Drugs that increase the pH of the upper gastrointestinal tract may decrease the solubility of nilotinib and reduce its bioavailability. The oral administration of esomeprazole resulted in a 34% reduction in the AUC of nilotinib.
Side effects
Nilotinib may cause anemia, neutropenia and thrombocytopenia. Prolonged QT interval and sudden death has occurred. Pruritus, rash, and nausea are common. Also seen with nilotinib are arthralgias and myalgias. Cough has also been associated with nilotinib.
Clinical evaluation
Nilotinib is the other second-generation Abl kinase inhibitor approved for treatment of patients with CML. Nilotinib has increased affinity for the Abl kinase compared with imatinib, binding with an improved topologic fit to the kinase site in its inactive form.Nilotinib is active in chronic and accelerated phase CML patients who have developed resistance to imatinib. As with dasatinib, nilotinib was compared with imatinib in a phase III randomized trial as initial therapy for chronic phase CML patients, with nilotinib achieving higher rates of complete cytogenetic and major molecular responses and with fewer cases of disease progression or clonal evolution in the nilotinib-treated cohorts. Survival outcomes were similar in all arms. Common adverse events with nilotinib included rash, gastrointestinal disturbances (nausea, vomiting, diarrhea), neutropenia, and thrombocytopenia. Pleural effusion and peripheral edema are less common than with dasatinib. In addition, QT prolongation and a risk of pancreatitis are serious side effects of nilotinib.
Description
Chronic myeloid leukemia (CML), a hematological stem-cell disorder, is definitively diagnosed by the detection of the Philadelphia chromosome, a truncated version of chromosome 22 resulting from the reciprocal translocation of chromosomes 9 and 22 induced by a single mutagenic event. The consequence is the juxtaposition of two genes creating a fusion gene BCR-ABL. This gene leads to the translation of a fusion protein with increased tyrosine kinase activity that contributes to the pathogenesis of CML. Targeting the BCR-ABL protein has led to the successful intervention of the disease. Now established as first-line therapy for CML, imatinib was the first selective tyrosine kinase inhibitor of BCR-ABL. Since imatinib only binds to an inactive conformation of the ABL kinase portion, the conformational restrictions contribute to its selectivity.
Chemical Properties
Off-White Solid
Originator
Novartis (Switzerland)
Uses
Nilotinib, an orally active signal transduction inhibitor that selectively inhibits the tyrosine kinase Bcr-Abl, was discovered and developed by Norvartis and was launched for the treatment of chronic myeloid leukemia (CML) in patients with Philadelphia chromosome-positive (Ph+) disease who are resistant or intolerant to imatinib mesilate. Additional clinical trials are currently underway for the treatment of acute lymphoblastic leukemia (ALL) and gastrointestinal stromal tumors (GISTs).
Uses
Nilotinib-d6, is the labeled analogue of Nilotinib, which might be useful in treatment of chronic myelogenous leukemia.
Uses
Nilotinib (AMN-107) is a Bcr-Abl inhibitor with IC50 less than 30 nM.
Definition
ChEBI: Nilotinib is a member of (trifluoromethyl)benzenes, a member of pyrimidines, a member of pyridines, a member of imidazoles, a secondary amino compound and a secondary carboxamide. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor and an anticoronaviral agent.
brand name
Tasigna
Clinical Use
Tyrosine kinase inhibitor:
Treatment of chronic myelogenous leukaemia
(CML)
Synthesis
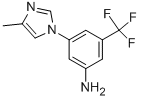
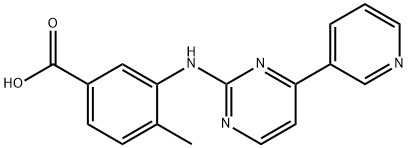
The first step in the synthesis of nilotinib involves the nucleophilic aromatic substitution of 3-fluoro-5-(trifluoromethyl)benzonitrile with 2-methylimidazole. The nitrile is then hydrolyzed with sodium hydroxide in aqueous dioxane. A Curtius rearrangement employing diphenylphosphoryl azide in tert-butanol affords the tert-butyl carbamate. Deprotection of the Boc group provides the 3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)aniline piece for the convergent synthesis. Construction of the other half begins with the condensation of 3-amino-4- methylbenzoic acid methyl ester with cyanamide in refluxing ethanolic HCl to generate the 3-guanidinobenzoate. An enamino ketone, prepared by a Claisen condensation of 3-acetylpyridine with ethyl formate in the presence of sodium metal in hot toluene, is then cyclized with the guanidine to yield the pyridylpyrimidine. Following saponification of the ethyl ester, the resultant 4-methyl-3-[4-(3-pyridyl)pyrimidin-2-ylamino]benzoic acid is finally coupled with the aniline utilizing diethyl cyanophosphate to provide nilotinib.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: avoid with clarithromycin, rifampicin
(concentration reduced) and telithromycin.
Antifungals: avoid with itraconazole, ketoconazole
(concentration increased) and voriconazole.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: avoid with boceprevir and ritonavir
(concentration possibly increased).
Grapefruit juice: avoid concomitant administration.
Avoid concomitant use with other inhibitors or
inducers of CYP3A4. Dose alterations may be
required.
Metabolism
Nilotinib is metabolised in the liver via oxidation and hydroxylation, in which cytochrome P450 isoenzyme CYP3A4 plays an important role. Most of an oral dose is eliminated unchanged in the faeces within 7 days.
References
1) Weisberg?et al.?(2006),?AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL; Br J. Cancer,?94?1765 2) Verstovsek?et al.?(2006),?Activity of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, against human FIP1L1-PDGFR-alpha-expressing cells; Ann. Neurol.,?75?209
Nilotinib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Yangzhou Qinyuan Pharmatech Co.,ltd | +86-18752526868 | jennysun@yzqyyykj.com | China | 64 | 58 |
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 | yuxin@yuxchem.com | China | 122 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Chembon Pharmaceutical Co., Ltd. | +86-28-8425-2981 | CHINA | 724 | 55 | |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9350 | 55 |
Related articles
- What is the difference between imatinib and nilotinib?
- Nilotinib, an orally bioavailable, selective Bcr-Abl tyrosine kinase inhibitor, is 30-fold more potent than imatinib in pre-cl....
- Mar 29,2024
- Nilotinib: Pharmacokinetics, Tolerability and Dosage
- Nilotinib, used in imatinib-resistant chronic myeloid leukaemia treatment, exhibits dose-dependent serum concentrations and go....
- Feb 21,2024
- Nilotinib: Uses, Side effects&Drug Interactions
- Nilotinib known as Tasigna is a targeted cancer drug. It is a treatment for chronic myeloid leukaemia (CML).
- Feb 15,2023
View Lastest Price from Nilotinib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-12 | Nilotinib
641571-10-0
|
US $0.00 / g | 1g | 98% HPLC | 1KG | shandong perfect biotechnology co.ltd | |
 |
2023-12-20 | Nilotinib
641571-10-0
|
US $0.00-0.00 / kg | 1kg | 99%,single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd | |
 |
2023-08-16 | Nilotinib
641571-10-0
|
US $30.00 / kg | 1kg | >99% | 300000kg | Weijer International Trade (Hebei) Co., Ltd |
641571-10-0(Nilotinib)Related Search:
1of4





