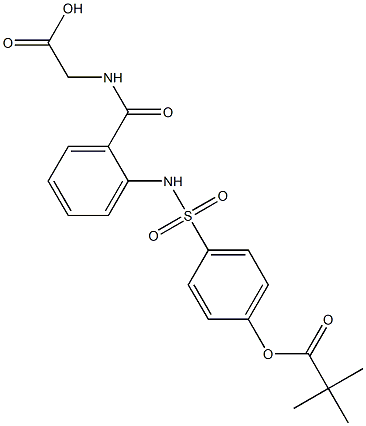
Adalimumab
- CAS No.
- 331731-18-1
- Chemical Name:
- Adalimumab
- Synonyms
- Adalimumab;Humira;D2E7;LU200134;Unii-fys6T7F842;Adalimumab Beta;Adalimumab (anti-TNF-α);Unii-fys6T7F842 USP/EP/BP;Adalimumab (anti-TNF-alpha);Research Grade Adalimumab(DHB94402)
- CBNumber:
- CB62494098
- Molecular Formula:
- C6428H9912N1694O1987S46
- Molecular Weight:
- 434.46288
- MDL Number:
- MFCD00162116
- MOL File:
- 331731-18-1.mol
| storage temp. | Store at -80°C |
|---|---|
| FDA UNII | FYS6T7F842 |
| NCI Drug Dictionary | adalimumab |
| ATC code | L04AB04 |
Adalimumab price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A-166 | Adalimumab(Humira)solution 10?mg/mL(12.5mMHistidineBuffer),certifiedreferencematerial,ampuleof0.2 | 331731-18-1 | 0.25ML | $849 | 2024-03-01 | Buy |
| Usbiological | 384854 | Adalimumab | 331731-18-1 | 96Tests | $1114 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | ATB0010215 | ADALIMUMAB 95.00% | 331731-18-1 | 5MG | $496.76 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FI139131 | Adalimumab | 331731-18-1 | 1mg | $500 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FI139131 | Adalimumab | 331731-18-1 | 2mg | $650 | 2021-12-16 | Buy |
Adalimumab Chemical Properties,Uses,Production
Description
Adalimumab is the first fully human neutralizing IgG1 monoclonal antibody specific for TNF-alpha and is the third TNF sequestrant marketed. It was launched in US, UK and Germany for the treatment of rheumatoid arthritis. It prevents TNF binding to p55 and p75 cell surface TNF receptors thereby decreasing leukocyte migration and acute phase reactants such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and levels of serum IL-6, MMP-1 and MMP-3. Adalimumab was developed starting with a phage-display derived murine antibody, followed by replacement of both heavy and light chains with human forms and further optimization to yield the final humanized form. In receptor binding studies, adalimumab exhibits an IC50 between 7.8×10-11 and 15.6×10-11M with a Kd of 1×10-10 M. It also binds to pro-TNFalpha on cell membranes mediating complement-dependent toxicity and to the Fc receptor mediating antibody-dependent cytotoxicity. In a human-TNF transgenic polyarthritis mouse model, adalimumab was efficacious based upon both clinical and histological readouts. Patients responding inadequately to methotrexate were co-administered methotrexate and adalimumab, which resulted in improved ACR scores in a 52-week study (ACR20: 59%; ACR50: 42%; ACR70; 23%). Radiographic analysis at six months showed decreased progression of structural joint damage (96%>placebo) and that joint-space narrowing stabilizes after six months. Adalimumab has an ED50 of 0.3 to 0.5 mg/kg and is dosed subcutaneously once every two weeks (0.8 mL containing 40 mg). The Vdss ranged from 0.063 to 0.76 L/kg consistent with being highly localized within the vasculature. It is slowly cleared with clearance values ranging from 0.18 to 0.27 mL/min and with a terminal half-life of about 12 days. As is the case with other TNF-alpha sequestrants, injection-site irritation is the most common side effect. The risk of developing opportunistic infection, especially tuberculosis, has been noted with TNF sequestrant biologics, which has led to screening of patients to identify those at risk.
Originator
Cambridge antibody technology (US)
Uses
Treatment of rheumatoid arthritis and other chronic inflammatory diseases (monoclonal antibody).
Definition
ChEBI: Sivelestat is a N-acylglycine and a pivalate ester. It is functionally related to a N-benzoylglycine.
Indications
Adalimumab has been evaluated in a number of clinical trials for RA, Crohn's disease,ankylosing spondylitis,and psoriatic arthritis. Initially evaluated as adjunctive therapy to RA patients on methotrexate, adalimumab demonstrated rapid improvement in American College of Rheumatology 20 scores at 1 week of administration. The PREMIER trial compared combination adalimumab plus methotrexate therapy with either medication given alone and found that the combination of adalimumab plus methotrexate was superior to adalimumab or methotrexate monotherapy.
brand name
Humira
Pharmacology
Adalimumab is a fully human, anti-TNF-α IgG1 monoclonal antibody, which blocks the interaction of TNF-α with p55 and p75 cell surface receptors.
Adalimumab is typically administered as a 20-or 40-mg dose via subcutaneous injection either weekly or every other week. The subcutaneous route of administration may be favorable to infliximab, which requires an intravenous infusion.
The terminal half-life of adalimumab ranges from 15 to 19 days and early phase I trials demonstrated no significant pharmacokinetic advantage to weight-based dosing strategies.
Clinical Use
Adalimumab is supplied in single-use, prefilled, glass syringes as a sterile, preservative-free, colorless solution for subcutaneous administration. The pharmacokinetics of adalimumab were linear over the dose range of 0.5 to 10.0 mg/kg following a single IV dose. The mean elimination half-life was approximately 2 weeks.
Side effects
Injection site reactions appear to be the most commonly reported adverse event and occur in up to 10% of patients treated. In an efficacy and safety study of adalimumab for ankylosing spondylitis, the number of adverse events was higher in patients receiving subcutaneous adalimumab 40 every other week than in those receiving placebo. The percentage of patients who experienced infectious complications was higher in the patients receiving adalimumab, but this finding was not statistically significant. No occurrences of latent tuberculosis reactivation, lupus-like syndromes, congestive heart failure, or secondary malignancies were reported.In a postmarketing surveillance study of RA patients, Schiff et al.reported that adalimumab appeared to be relatively safe and well tolerated. In their study, safety data from randomized clinical trials, open-label extensions, phase IIIb trials, and postmarketing reporting of adverse events in the USA were collected. Reported adverse events included serious infections (5.1 events/100 patient-years (PYs)), lymphoma (0.12/100 PYs), tuberculosis (0.27/100 PYs), opportunistic infections (0.08 events/100 PYs), demyelinating diseases (0.08/100 PYs), systemic lupus erythematosis/lupus-like syndrome (0.10/100 PYs), and congestive heart failure (0.28/100 PYs). The incidence of lymphoma did not appear to be significantly higher in patients treated with adalimumab than in RA patients who were naïve to anti-TNF-α therapy; however, the rate of lymphoma may be higher in RA patients compared to the general population, particularly in patients with severe RA. Adverse events reported in patients with ophthalmic inflammatory disease treated with adalimumab have included injection site reactions, herpes simplex keratitis, and elevation of liver enzymes requiring cessation of therapy.
Drug interactions
Adalimumab is currently approved for RA and psoriatic arthritis in combination with methotrexate and low-dose prednisone. Live viruses should be avoided in patients on adalimumab and its use may decrease the immunologic protection conferred by live attenuated vaccines. No clear data are available for its use in combination with other biologic agents, so this combination should be avoided until further studies have demonstrated efficacy and safety.
Metabolism
Most likely removed by opsonisation via the reticuloendothelial system.
Precautions
Adalimumab is contraindicated in patients with known hypersensitivity to the medication or any of its components and in patients at risk for sepsis. In addition, the medication should be avoided in patients with a history of multiple sclerosis, active infection, or malignancy.
Adalimumab Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Minbiotech Co., Ltd. | +8617315815539 | sales@minbiotech.com | CHINA | 129 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3632 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3465 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29271 | 58 |
Related articles
- The mechanism of action and storage method of Unii-fys6T7F842
- Unii-fys6T7F842 is a revolutionary biological drug that has become a key therapeutic choice for various autoimmune diseases.
- Apr 10,2024
- The introduction of Unii-fys6T7F842
- The Unii-fys6T7F842, with the CAS No: 331731-18-1, is the first human anti-tumor necrosis factor approved for marketing in the....
- Feb 3,2023
View Lastest Price from Adalimumab manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-02-04 | Adalimumab
331731-18-1
|
US $0.00 / G | 1G | 98%min | 30kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-09-16 | Adalimumab
331731-18-1
|
US $0.00-0.00 / g | 10mg | 99% | Adalimumab | Hangzhou Huarong Pharm Co., Ltd. | |
 |
2021-07-20 | Adalimumab
331731-18-1
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd |
-

- Adalimumab
331731-18-1
- US $0.00 / G
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Adalimumab
331731-18-1
- US $0.00-0.00 / g
- 99%
- Hangzhou Huarong Pharm Co., Ltd.
-

- Adalimumab
331731-18-1
- US $1.00-1.00 / KG
- 99%
- Shaanxi Dideu Medichem Co. Ltd