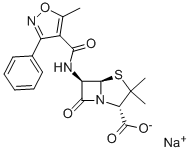
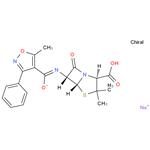
OXACILLIN SODIUM
- CAS No.
- 1173-88-2
- Chemical Name:
- OXACILLIN SODIUM
- Synonyms
- p12;OXACILLIN SODIUM SALT;bactocill;prostaphlin;SODIUM OXACILLIN;Oxacillin sodiuM anhydrous;OXACILLIN SODIUM SALT HYDRATE;7-[[3-(2-chlorophenyl)-5-methyl-oxazol-4-yl]carbonylamino]-3,3-dimethyl-6-oxo-2-thia-5-azabicyclo[3.2.0]heptane-4-carboxylic acid sodium salt monohydrate;oxabel;brl1400
- CBNumber:
- CB6278850
- Molecular Formula:
- C19H18N3NaO5S
- Molecular Weight:
- 423.42
- MDL Number:
- MFCD00056864
- MOL File:
- 1173-88-2.mol
| Melting point | 188°C |
|---|---|
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: soluble50mg/mL |
| form | crystalline |
| color | colorless or white |
| BRN | 4098179 |
| FDA UNII | 4TWD2995UP |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H334 | |||||||||
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XH9100000 | |||||||||
| Toxicity | cat,LDLo,oral,> 750mg/kg (750mg/kg),Arzneimittel-Forschung. Drug Research. Vol. 15, Pg. 322, 1965. | |||||||||
| NFPA 704 |
|
OXACILLIN SODIUM price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 28221 | Oxacillin sodium salt ~95% (TLC) | 1173-88-2 | 1g | $44.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 28221 | Oxacillin sodium salt ~95% (TLC) | 1173-88-2 | 5g | $108 | 2024-03-01 | Buy |
| Cayman Chemical | 23954 | Oxacillin (sodium salt) ≥98% | 1173-88-2 | 1g | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 23954 | Oxacillin (sodium salt) ≥98% | 1173-88-2 | 5g | $90 | 2024-03-01 | Buy |
| Cayman Chemical | 23954 | Oxacillin (sodium salt) ≥98% | 1173-88-2 | 10g | $161 | 2024-03-01 | Buy |
OXACILLIN SODIUM Chemical Properties,Uses,Production
Brand Name(s) in US
Pro-staphli, and generic
Chemical Properties
Crystalline
Originator
Resistopen,Squibb,US,1962
Uses
Oxacillin sodium salt is a narrow spectrum beta-lactam antibiotic. It can be used in analytical study of combination of reversed phase liquid chromatography and zwitterion exchange-reversed phase-hydrophilic interaction mixed-mode liquid chromatography coupled with mass spectrometry for anal. of antibiotics and their impurities.
Uses
Semi-synthetic antibiotic related to Penicillin. Antibacterial
Definition
ChEBI: Oxacillin sodium is an organic sodium salt. It contains an oxacillin(1-).
Manufacturing Process
(A) Benzaldoxime: (Reference, Vogel, Textbook of Practical Organic Chemistry, page 883) -Materials: (Theoretical yield, 121.1 grams of free oxime), 106.1 grams (1.0 mol) of benzaldehyde (NF grade), 69.5 grams (1.0 mol) of hydroxylamine hydrochloride (practical grade), 68.0 grams (1.7 mol) of sodium hydroxide (pellet).
Procedure: The sodium hydroxide is dissolved in 200 ml water and the benzaldehyde is added. With continued stirring the hydroxylamine hydrochloride is added in portions. Some heat is developed and eventually the benzaldehyde dissolves. The solution is stirred for 15 minutes and then cooled in an ice-bath. A waxy, crystalline mass separates, and after further cooling it is collected by suction and dried in air. Yield is 86 to 149 grams. This crude material is suitable for step (B).
(B) Benzohydroximic Chloride: [Reference, G.W. Perrold et al, J. Am. Chem. Soc., 79, 462 (1957)] - Materials: 121 grams (0.77 mol) of crude benzaldoxime from step (A), 500 ml of 8.3 N hydrochloric acid, chlorine.
Procedure: The crude product from (A) is suspended in the hydrochloric acid, cooled in an ice-salt mixture, and chlorine is passed into the mixture with stirring for ? to 1 hour. Transient blue and green colors may be noticed in the mixture during this time. The temperature will probably rise to 3° to 5°C. The solid is collected by suction filtration and dried for an hour or so on the filter before use in (C). If at all possible, it should be used on the day of preparation. Yield is 71 grams (after 1? hours on the filter).
(C) 5-Methyl-3-Phenyl-4-Isoxazolecarboxylic Acid: [Reference, A. Quilico and R. Rusco, Gazz. Chim. Ital. 67, 589 (1937); C.A. 32, 21177] - Materials: 71 grams (0.45 mol) of crude benzohydroximic chloride from (E), 78 grams (0.60 mol) of ethyl acetoacetate (practical grade), 34 grams (0.60 mol) of sodium methoxide (95% minimum), 400 ml of methanol (reagent grade).
Procedure: The sodium methoxide is cautiously added in portions to 200 ml of methanol with stirring. Some heat is evolved. To this warm solution is rapidly added the ethyl acetoacetate with continued stirring. The solution is stirred for 10 minutes and then cooled in an ice-salt-acetone mixture (-25°C). If desired a Dry Ice-acetone cooling bath may be used to shorten the addition time. The crude material from (B) is dissolved in 200 ml of methanol. At this point it is probably easier to filter this mixture by suction to remove a large amount of insoluble solid, which is probably sodium chloride. The solid may be rinsed with more methanol.
The filtrate is chilled in ice-water and added to the cooled methanolic solution of the sodium derivative of ethyl acetoacetate at a rate which keeps the temperature of the re. action mixture below 0°C. The addition time will be 15 to 20 minutes if ice-salt-acetone is used as a coolant. This reaction is extremely exothermic.
The reaction mixture is stirred overnight at room temperature and filtered to remove the sodium chloride. The filtrate is stripped in vacuo and the crude ester (literature reports MP 48°C) is dissolved in 150 ml of ethanol; 28 grams (0.70 mol of sodium hydroxide in 90 ml of water is added and the solution is refluxed for 2 hours. After removal of the ethanol in vacuo the residue is dissolved in water and extracted twice with ether. Dissolved ether is removed from the aqueous solution in vacuo and it is acidified to pH 2 with concentrated hydrochloric acid.
The crystalline crude acid is dried briefly and then recrystallized from acetonitrile to give 32 grams of white product; MP 193° to 194.5°C (literature reports 189° to 190°C). Concentration of the mother liquor gives an additional 5 grams of material having a MP of 192.5 to 194°C. The 37 grams of material represents an 18% overall yield from benzaldehyde.
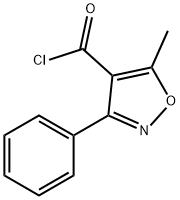
(D) The acid is converted to the acid chloride by reaction with thionyl chloride.
(E) 5-Methyl-3-Phenyl-4-Isoxazolylpenicillin: A solution of 4.43 grams of 5methyl-3-phenylisoxazole-4-carbonyl chloride in 120 ml acetone was added
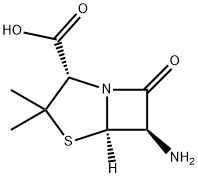
gradually to a stirred solution of 4.32 grams of 6-aminopenicillanic acid in 168 ml of 3% aqueous sodium bicarbonate and 50 ml acetone. When addition was complete the mixture was stirred at room temperature for 4 hours and then extracted with ether (2 x 200 ml), only the aqueous phase being retained. This aqueous solution was covered with 50 ml ether and adjusted to pH 2 by the addition of N hydrochloric acid. After separating the layers, the aqueous phase was extracted with two further 50 ml portions of ether. The combined ether solutions (which at this stage contained the free penicillin acid) were washed with water and then neutralized by shaking with 20 ml N sodium bicarbonate solution. The aqueous phase was separated, washed with ether, and evaporated at low temperature and pressure to leave the crude sodium salt of 5-methyl-3-phenyl-4-isoxazolylpenicillin as a white solid, which was finally dried in vacuo over phosphorus pentoxide and found to weigh 7.34 grams.
brand name
Bactocill (GlaxoSmithKline); Prostaphlin (Apothecon).
Therapeutic Function
Antibacterial
Clinical Use
Oxacillin sodium, (5-methyl3-phenyl-4-isoxazolyl)penicillinsodium monohydrate (Prostaphlin), is the salt of a semisyntheticpenicillin that is highly resistant to inactivation bypenicillinase. Apparently, the steric effects of the 3-phenyland 5-methyl groups of the isoxazolyl ring prevent the bindingof this penicillin to the β-lactamase active site and,thereby, protect the lactam ring from degradation in muchthe same way as has been suggested for methicillin. It is alsorelatively resistant to acid hydrolysis and, therefore, may beadministered orally with good effect.
Oxacillin sodium, which is available in capsule form, isreasonably well absorbed from the gastrointestinal (GI)tract, particularly in fasting patients. Effective plasma levelsof oxacillin are obtained in about 1 hour, but despite extensiveplasma protein binding, it is excreted rapidly throughthe kidneys. Oxacillin experiences some first-pass metabolismin the liver to the 5-hydroxymethyl derivative. Thismetabolite has antibacterial activity comparable to that ofoxacillin but is less avidly protein bound and more rapidlyexcreted. The halogenated analogs cloxacillin, dicloxacillin,and floxacillin experience less 5-methyl hydroxylation.
The use of oxacillin and other isoxazolylpenicillinsshould be restricted to the treatment of infections causedby staphylococci resistant to penicillin G. Although theirspectrum of activity is similar to that of penicillin G, theisoxazolylpenicillins are, in general, inferior to it and thephenoxymethylpenicillins for the treatment of infectionscaused by penicillin G-sensitive bacteria. Because isoxazolylpenicillinscause allergic reactions similar to thoseproduced by other penicillins, they should be used withgreat caution in patients who are penicillin sensitive.
Purification Methods
This antibiotic, which is stable to penicillinase, is purified by recrystallisation from isoPrOH and dried in vacuo. Its solubility in H2O at 25o is 5%. [Doyle et al. Nature 192 1183 1961, Review: Chain et al. Antibiotics (Oxford University Press) 2 1949.]
OXACILLIN SODIUM Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9350 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3684 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
View Lastest Price from OXACILLIN SODIUM manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Oxacillin Sodium
1173-88-2
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC | |
 |
2023-11-01 | Oxacillin sodium salt
1173-88-2
|
US $0.00-0.00 / kg | 1kg | 99.0% | 20 tons | Hangzhou ICH Biofarm Co., Ltd | |
 |
2023-07-19 | OXACILLIN SODIUM
1173-88-2
|
US $10.00 / KG | 1KG | 99% | 100MT/Month | Wuhan wingroup Pharmaceutical Co., Ltd |
-

- Oxacillin Sodium
1173-88-2
- US $0.00-0.00 / mg
- 90%+
- Guangzhou PI PI BIOTECH INC
-

- Oxacillin sodium salt
1173-88-2
- US $0.00-0.00 / kg
- 99.0%
- Hangzhou ICH Biofarm Co., Ltd
-

- OXACILLIN SODIUM
1173-88-2
- US $10.00 / KG
- 99%
- Wuhan wingroup Pharmaceutical Co., Ltd





