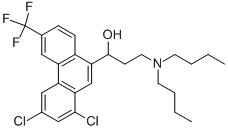
HALOFANTRINE
- CAS No.
- 69756-53-2
- Chemical Name:
- HALOFANTRINE
- Synonyms
- Halofan;WR-171669;SKF-102886;HALOFANTRINE;Halofantrine D18;HALOFANTRINE USP/EP/BP;Halofantrine hydrochloride CRS;3-(Dibutylamino)-1-(1,3-dichloro-6-(trifluoromethyl)-phenanthren-9-yl)propan-1-ol;1,3-Dichloro-α-[2-(dibutylamino)ethyl]-6-(trifluoromethyl)-9-phenanthrenemethanol;3-DIBUTYLAMINO-1-(1,3-DICHLORO-6-TRIFLUOROMETHYL-PHENANTHREN-9-YL)-PROPAN-1-OL HCL
- CBNumber:
- CB6377605
- Molecular Formula:
- C26H30Cl2F3NO
- Molecular Weight:
- 500.42
- MDL Number:
- MFCD00866195
- MOL File:
- 69756-53-2.mol
| Density | 1.244±0.06 g/cm3 (20 ºC 760 Torr) |
|---|---|
| storage temp. | 2-8°C |
| Water Solubility | 0.59mg/L(37 ºC) |
| FDA UNII | Q2OS4303HZ |
| ATC code | P01BX01 |
HALOFANTRINE price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| AK Scientific | K173 | HalofantrineHCl | 69756-53-2 | 5mg | $74 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0002883 | HALOFANTRINE 95.00% | 69756-53-2 | 10MG | $216.3 | 2021-12-16 | Buy |
| AK Scientific | K173 | HalofantrineHCl | 69756-53-2 | 25mg | $278 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0002883 | HALOFANTRINE 95.00% | 69756-53-2 | 1G | $945.95 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 18652 | Halofantrine | 69756-53-2 | 5mg | $950 | 2021-12-16 | Buy |
HALOFANTRINE Chemical Properties,Uses,Production
Pharmacology and mechanism of action
Halofantrine is a phenanthrenemethanol antimalarial drug developed by the US military. The antimalarial activity of the phenanthrenemethanols was discovered during the Second World War but was not exploited until later. Halofantrine is a potent blood schizontocide against Plasmodium falciparum both in vitro and in vivo. However, it has no effect against exoerythrocytic forms of the parasite. Experience of its activity against other malaria species is limited. The drug exists as a racemic mixture, but the two enantiomers have shown similar activity in vitro [1, 2].
The mechanism of action of halofantrine is not known.
Indications
Halofantrine is indicated only for the treatment of multidrug-resistant Plasmodium falciparum malaria.
Side effects
Halofantrine is generally well tolerated. Mild and transient side effects such as nausea, vomiting, diarrhoea, abdominal pain, pruritus and rash have been reported in humans. Halofantrine is potentially cardiotoxic particularly with doses above the recommended dose and causes ECG changes such as prolongation of PR and QTc intervals. In one study, the sudden death of a patient was reported after receiving a high dose of halofantrine (8 mg/kg 3 times daily for 3 days) [3]. The patient had previously been treated with mefloquine. Cardiotoxicity due to halofantrine will become a therapeutic problem if higher dosage regimens have to be used due to decreased efficacy [3]. Occasional elevation of serum transaminase have been observed in some patients. The relationship of this to the treatment is unclear. Values usually return to normal levels within a week after treatment [4-6].
Contraindications and precautions
Halofantrine should not be given to patients with pre-existing cardiovascular diseases. There is a warning against the concomitant intake of any cardiotoxic drugs. Halofantrine is not used for malaria prophylaxis.
Interactions
There are no reports of interactions [7].
Preparations
Available as halofantrine hydrochloride: 100 mg hydrochloride is equal to 93 mg base. Not yet available for parenteral use.
• Halfan® (SmithKline & Beecham). Tablets 250 mg. Oral suspension 20 mg/ml.
References
1. Bryson HM, Goa KL (1992). Halofantrine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic potential. Drugs, 43, 236–258.
2. Karle JM, Olmeda R, Gerena L, Milhous WK (1994). Plasmodium falciparum: Role of absolute stereochemistry in the antimalarial activity of synthetic aminoalcohol antimalarial agents. Exp Parasitol, 76, 345–351.
3. Nosten F, ter Kuile FO, Luxemburger C, Woodrow C, Kyle DPE, Chongsuphajaisiddhi T, White NJ (1993). Cardiac effects of antimalarial treatment with halofantrine. Lancet, 341, 1054–1056.
4. Watkins WM, Lury JD, Kariuki D, Koech DK, Oloo JA, Mosoba M, Mjomba M, Gilles HM (1988). Efficacy of multiple-dose halofantrine in treatment of chloroquine-resistant falciparum malaria in children in Kenya. Lancet, 2, 247–250.
5. Salako LA, Sowunmi A, Walker O (1990). Evaluation of the Clinical trials and safety of halofantrine in falciparum malaria in Ibadan Nigeria. Trans R Soc Trop Med Hyg, 84, 644–647.
6. Boudreau EF, Pang LW, Dixon KE, Webster HK, Pavanand K, Tosingha L, Somutsakorn P, Canfield CJ (1988). Malaria: Treatment efficacy of halofantrine (WR171,669) in initial field trials in
7. Karbwang J, Na Bangchang K (1994). Clinical pharmacokinetics of halofantrine. Clin Pharmacokinet, 27(2), 104–119.
Description
Halofantrine is an orally-active blood schizonticide reportedly highly effective in the treatment of fulcipurum malaria and other types of parasitemia. Cure rate is claimed to be over 95%.
Originator
Smith mine & French (USA)
Definition
ChEBI: 3-(dibutylamino)-1-[1,3-dichloro-6-(trifluoromethyl)-9-phenanthrenyl]-1-propanol is a member of phenanthrenes.
brand name
Halfan
World Health Organization (WHO)
Halofantrine is an antimalarial introduced to medicine in 1982. It should be reserved for use in areas where multiple drug-resistant falciparum malaria is prevalent. Cases of serious cardiotoxicity have been reported.
Antimicrobial activity
It inhibits erythrocytic stages of chloroquine-sensitive and chloroquine-resistant P. falciparum and other Plasmodium spp. in vitro at concentrations of 0.4–4.0 mg/L. It is more active than mefloquine and the combination of proguanil and atovaquone against P. falciparum, but less effective than mefloquine or chloroquine against P. vivax.
Acquired resistance
Resistance in P. falciparum has been reported in Central and West Africa, where it has been used widely. Cross-resistance with mefloquine has been reported in Thailand, where it has not been used.
General Description
Structurally, halofantrine differs from allother antimalarial drugs. It is a good example of drug designthat incorporates bioisosteric principles as evidenced by thetrifluromethyl moiety. Halofantrine is a schizonticide and has no affect on the sporozoite, gametocyte,or hepatic stages. Both the parent compound and Ndesbutylmetabolite are equally active in vitro. Halfantrine’sspecific mechanism of action against the parasite is notknown. There is contradictory evidence that its mechanismranges from requiring heme to disrupting the mitochondria.There is a prominent warning that halfantrine can affectnerve conduction in cardiac tissue.
Pharmaceutical Applications
A phenanthrene methanol, formulated as the hydrochloride for oral administration. Parenteral formulations are not available. The enantiomers have equivalent activity in vitro. Aqueous solubility is extremely low.
Pharmacokinetics
Absorption shows high intra- and inter-subject variability and depends on co-administration with fats. Bioavailability is increased more than six-fold after a fatty meal or by lipidbased formulations. Bioavailability is significantly lower in patients with malaria than in healthy individuals. Peak plasma levels are variable and occur 3–6 h after administration. Unlike many other antimalarials, halofantrine is not concentrated by infected or uninfected erythrocytes. Distribution to lipoproteins is stereo-selective. About 20–30% of the dose is metabolized to an N-desbutyl derivative by cytochrome P450 (CYP) 3A4 and 3A5. The elimination half-life of the parent drug is generally 1–2 days and that of the metabolite 3 days. Little unchanged drug is excreted in urine.
Clinical Use
Treatment of multidrug-resistant falciparum malaria
Its use has been questioned due to the existence of safer
alternatives.
Side effects
Abdominal pain, diarrhea and pruritus are the most frequent. High doses (24 mg/kg) induce prolongation of the PR and QTc intervals; this is not stereo-selective. There are individual reports of fatal cardiac arrest and torsade de pointes. To reduce the risk of cardiac toxicity it should be taken on an empty stomach. It should not be administered with other antimalarials that have the potency to induce cardiac arrhythmias (mefloquine, chloroquine, quinine). Halofantrine has also been associated with intravascular hemolysis.
Metabolism
Halofantrine is considered to be an alternative drug for treatment of both chloroquine-sensitive and chloroquine-resistant Plasmodium falci parum malaria, but its efficacy in mefloquine-resistant malaria may be questionable. The drug is metabolized via N-dealkylation to desbutylhalofantrine by CYP3A4. The metabolite appears to be several-fold more active than the administered drug.
HALOFANTRINE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | 1015@dideu.com | China | 2263 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29474 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| Pure Chemistry Scientific Inc. | 001-857-928-2050 or 1-888-588-9418 | sales@chemreagents.com | United States | 10194 | 62 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2127 | 70 |
| Shanghai Fuhe Chemistry Technology Co., Ltd. | 0086-21-67651709 | cfx759@hotmail.com | China | 410 | 57 |
View Lastest Price from HALOFANTRINE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-06-18 | HALOFANTRINE USP/EP/BP
69756-53-2
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited | |
 |
2020-04-28 | Halofantrine
69756-53-2
|
US $0.00-0.00 / Kg | 1KG | 99.0% | 500 tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2019-12-31 | HALOFANTRINE
69756-53-2
|
US $1.00 / KG | 1KG | 95-99% | 1ton | Career Henan Chemical Co |
-

- HALOFANTRINE USP/EP/BP
69756-53-2
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Halofantrine
69756-53-2
- US $0.00-0.00 / Kg
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
-

- HALOFANTRINE
69756-53-2
- US $1.00 / KG
- 95-99%
- Career Henan Chemical Co
69756-53-2(HALOFANTRINE)Related Search:
1of4