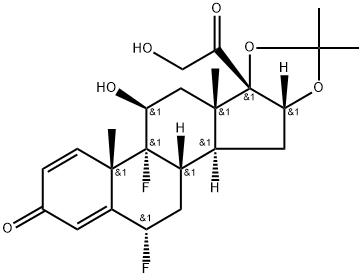
fluocinolone16,17-acetonide
- CAS No.
- 67-73-2
- Chemical Name:
- fluocinolone16,17-acetonide
- Synonyms
- synalar;Fluonid;dermalar;Fluocinolone acetonide for system suitability;6-alpha,9-alpha-difluoro-16-alpha-hydroxyprednisolone16,17-acetonide;jellin;synsac;Fluzon;localyn;synamol
- CBNumber:
- CB6394372
- Molecular Formula:
- C24H30F2O6
- Molecular Weight:
- 452.49
- MDL Number:
- MFCD00010525
- MOL File:
- 67-73-2.mol
| Melting point | 267-269 °C(lit.) |
|---|---|
| Boiling point | 578.5±50.0 °C(Predicted) |
| alpha | D +95° (chloroform) |
| Density | 1.1826 (estimate) |
| refractive index | 103 ° (C=1, MeOH) |
| storage temp. | Refrigerator |
| solubility | Practically insoluble in water, soluble in acetone and in ethanol |
| pka | 12.78±0.70(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble in water (partly), DMSO, alcohol, chloroform and methanol. |
| Merck | 14,4150 |
| InChIKey | FEBLZLNTKCEFIT-VSXGLTOVSA-N |
| CAS DataBase Reference | 67-73-2(CAS DataBase Reference) |
| FDA UNII | 0CD5FD6S2M |
| ATC code | C05AA10,D07AC04,S01BA15,S02BA08 |
| EPA Substance Registry System | Fluocinolone acetonide (67-73-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-40-20/21/22 | |||||||||
| Safety Statements | 26-36-22 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TU3830000 | |||||||||
| HS Code | 2937220000 | |||||||||
| Toxicity | LD50 oral in rat: > 4gm/kg | |||||||||
| NFPA 704 |
|
fluocinolone16,17-acetonide price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | F8880 | Fluocinolone acetonide analytical standard | 67-73-2 | 1g | $553 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1275009 | Fluocinolone acetonide United States Pharmacopeia (USP) Reference Standard | 67-73-2 | 100mg | $447 | 2024-03-01 | Buy |
| Alfa Aesar | J63996 | Fluocinolone acetonide | 67-73-2 | 100mg | $55.7 | 2023-06-20 | Buy |
| Alfa Aesar | J63996 | Fluocinolone acetonide | 67-73-2 | 1g | $294 | 2023-06-20 | Buy |
| Cayman Chemical | 23027 | Fluocinolone acetonide ≥98% | 67-73-2 | 500mg | $106 | 2024-03-01 | Buy |
fluocinolone16,17-acetonide Chemical Properties,Uses,Production
Corticosteroid drug
Fluocinolone acetonide is a kind of topical corticosteroids of the most significant effect and the least side effect in current china, and can cause the contraction of dermal capillary, inhibit cell proliferation or regeneration of connective tissue; it can also stabilize the intracellular lysosomal membrane and prevent the release of histamine from the intracellular lysosomal enzyme and the further tissue damage. The anti-inflammatory effects of Fluocinolone is 100 times as great s that of hydrocortisone. It has significant efficacy even at the lowest concentration (0.025%) with rapid onset rate and can significantly reduce or cure the symptoms in a few days with a relative excellent itching relief effect.

Fluocinolone acetonide is mainly used for the treatment of skin diseases to which glucocorticoid is effective such as contact dermatitis, atopic dermatitis, seborrheic dermatitis, neurodermatitis, dermatitis, eczema (especially infant eczema), pruritus disease, psoriasis, discoid lupus erythematosus, lichen planus, otitis externa and so on. Its combination with neomycin and other antibiotics can be used for the treatment of suppurative skin disease. Apply 0.025-0.05% ointment, lotion for scrubbing the affected area with 2-3 times per day. You can gently knead and make it be incorporated into the skin.
Physical and Chemical Properties
It appears as white or white-like crystalline powder and is odorless and tasteless. [α] D20+80-+88° (dioxane). It is soluble in acetone, slightly soluble in ethanol, dioxane but insoluble in water and petroleum ether. It is obtained through the sulfonic esterfication, ring-opening reaction and further 16-steps reactions starting from the 11α-hydroxy-16α, 17α-epoxy pregnane-4-ene-3, 20-dione. It can be applied to atopic dermatitis, contact dermatitis, eczema and other skin diseases.
Fluocinolone acetonide
Fluocinolone acetonide is a synthetic, fluorine-containing potent steroid. Topical administration of it can cause dermal capillary contraction and can inhibit epidermal cell proliferation or regeneration, inhibit the newborn of the fibroblast cells inside the connective tissue and stabilize the intracellular lysosomal membrane with anti-inflammatory and anti-itching effect. After topical administration, it can be absorbed through intact skin. After its absorption, similar as the metabolism of corticosteroid after systemic administration, it is mainly metabolized in the liver and can be excreted by the kidneys. Fluocinolone acetonide is suitable for treating the skin disease to which corticosteroid is effective skin such as eczema (especially eczema), neurodermatitis, skin pruritus, contact dermatitis, psoriasis, discoid lupus erythematosus, lichen planus, otitis externa, and solar dermatitis. However, it should be note that long-term or large-scale application can cause skin atrophy, telangiectasia and the occurrence of acne-like dermatitis, perioral dermatitis, folliculitis, and increased susceptibility to infection and so on. Allergic contact dermatitis can also occur in some cases.
This information is edited by Xiongfeng Dai from Chemicalbook.
Uses
It is suitable in the treatment of eczema, neurodermatitis, skin pruritus, contact dermatitis, psoriasis, dermatitis and other diseases
Chemical Properties
Crystalline Solid
Originator
Synalar,Syntex,US,1961
Uses
A synthetic glucocorticoid VEGF inhibitor; anti-inflammatory.
Uses
Fluocinolone acetonide is primarily used in dermatology to reduce skin inflammation and relieve itching. It acts as an inhibitor of tumor cells and it can regulate lipid metabolism by modulating gene expression. It can also inhibit the expression of VEGF (vascular endothelial growth factor) in the retinal pigment epithelial cell line due to its high glucocorticoid receptor affinity. Furthermore, it can inhibit TNF-α angiogenesis.
Uses
Fluocinolone acetonide (Derma-Smoothe/FS, Flurosyn, Capex, Synalar) is a synthetic fluorinated corticosteroid.
Definition
ChEBI: A fluorinated steroid that is flunisolide in which the hydrogen at position 9 is replaced by fluorine. A corticosteroid with glucocorticoid activity, it is used (both as the anhydrous form and as the dihydrate) in creams, gels and ointments for the treatme t of various skin disorders.
Indications
Fluocinolone acetonide (Derma-Smoothe/FS, Flurosyn, Capex, Synalar) is a synthetic fluorinated corticosteroid.
Manufacturing Process
A mixture of 1.2 grams of 6α-fluoro-16α-hydroxy-hydrocortisone, 4 cc of
acetic anhydride and 8 cc of pyridine was heated at 60°C for 2 hours and then
kept at room temperature for 2 hours. Ice and water were added and the
solid was collected, washed with water, dried and recrystallized from
methylene chloride-methanol, thus giving 1.05 grams of the 16,21-diacetate
of 6α-fluoro-16α-hydroxy-hydrocortisone (solvated) of MP 182° to 187°C;concentration of the mother liquors afforded an additional 130 mg of the same
compound, MP 184° to 187°C. By recrystallization from the same solvents
there was obtained the compound with a lower constant melting point of 175°
to 177°C.
2.94 grams of the 16,21-diacetate of 6α-fluoro-16α-hydroxy-hydrocortisone
was mixed with 60 cc of dimethylformamide, 3.6 cc of pyridine and 2.4 cc of
methane-sulfonyl chloride was heated on the steam bath for 2 hours. The
diacetate of 6α-fluoro-16α-hydroxy-hydrocortisone had been prepared as set
forth above, and further dried by azeotropic distillation with benzene; the
dimethylformamide had been previously distilled. After the 2 hours on the
steam bath the mixture was cooled and poured into saturated aqueous
sodium bicarbonate solution; the product was extracted with methylene
chloride, the extract was washed with water, dried over anhydrous sodium
sulfate and the solvent was evaporated.
The residue was chromatographed on 90 grams of silica gel eluting the
product with methylene chloride-acetone (9:1) and then recrystallizing from
methylene chloride-methanol. There was thus obtained 1.6 grams of the
16,21-diacetate of 6α-fluoro-δ4,9(11)-pregnadiene-16α,-17α,21-triol-3,20-dione
with MP 110° to 114°C; the analytical sample melted at 115° to 117°C,
[α]D+23.5° (chloroform), λ max. 234 to 236 nm, log ε 4.18.
A mixture of 1.38 grams of the above compound and 15 cc of dioxane was
treated with 1.9 cc of a 0.5 N aqueous solution of perchloric acid and 600 mg
of N-bromoacetamide, adding the latter in the dark, in three portions, in the
course of half an hour and under continuous stirring, It was then stirred for a
further 1% hours in the dark, then the excess of reagent was decomposed by
the addition of aqueous sodium bisulfite solution and ice water was added; the
product was extracted with methylene chloride, washed with water, dried over
anhydrous sodium sulfate and the solvent was evaporated under reduced
pressure, thus giving a yellow oil consisting of the 16,21-diacetate of 6α-
fluoro-9α-bromo-16α-hydroxy-hydrocortisone which was used for the next
step without further purification.
The above crude bromohydrin was mixed with 2.5 grams of potassium acetate
and 60 cc of acetone and refluxed for 6 hours, at the end of which the
acetone was distilled, water was added to the residue and the product was
extracted with methylene chloride. The extract was washed with water, dried
over anhydrous sodium sulfate and the solvent was evaporated.
Recrystallization of the residue from methanol furnished 800 mg of the 16,21-
diacetate of 6α-fluoro-9β,11β-oxido-δ4-pregnene-16α,17α,21-triol-3,20-dione
with MP 120° to 124°C; by chromatography of the mother liquors on silica gel
there was obtained 180 milligrams more of the same compound with MP 117°
to 119°C. The analytical sample was obtained by recrystallization from
methanol; it showed MP 125° to 127°C.
To a solution of 1.6 grams of anhydrous hydrogen fluoride in 2.85 grams of
tetrahydrofurane and 10 cc of methylene chloride cooled to -60°C was added
a solution of 650 mg of the 16,21-diacetate of 6α-fluoro-9β,11β-oxido-δ4-
pregnene-16α,17α,21-triol-3,20-dione in 20 cc of methylene chloride and the
mixture was kept at -10°C for 72 hours. It was then poured into saturated
aqueous sodium bicarbonate solution and the organic layer was separated,
washed with water, dried over anhydrous sodium sulfate and evaporated. The residue was reacetylated by heating with 3 cc of acetic anhydride and 6 cc of
pyridine for 1 hour on the steam bath. The reagents were evaporated under
reduced pressure and the residue was chromatographed on 30 grams of silica
gel. Upon elution with methylene chloride-acetone (9:1) and recrystallization
of the residue from methylene chloride-methanol there was obtained 290 mg
of the 16,21-diacetate of 6α,9α-difluoro-16α-hydroxy-hydrocortisone which
melted with loss of solvent at 140° to 150°C. Recrystallization from acetonehexane
afforded the analytical sample which was dried at 130°C; it then
showed a MP of 182° to 185°C.
brand name
Fluonid (Allergan); Fluotrex (Savage); FS Shampoo (Galderma); Retisert (Bausch & Lomb); Synalar (Medicis).
Therapeutic Function
Glucocorticoid, Antiinflammatory
General Description
Fluocinolone acetonide,6α,9-difluoro-11β,21-dihydroxy-16α,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione, also known as6α-fluorotriamcinolone acetonide, is the 21-acetate derivativeof fluocinolone acetonide and is about 5 times morepotent than fluocinolone acetonide in at least one topicalactivity assay.
fluocinolone16,17-acetonide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 | sales@chembj.net | China | 269 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 | nova@sh-teruiop.com | China | 525 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | Jany1001@kangcang.com.cn | China | 338 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
Related articles
- Fluocinolone acetonide-Application
- Fluocinolone acetonide is a fluorinated steroid that is flunisolide in which the hydrogen at position 9 is replaced by fluorin....
- Dec 12,2019
View Lastest Price from fluocinolone16,17-acetonide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Fluocinolone Acetonide
67-73-2
|
US $20.00-10.00 / kg | 1kg | 98% | 20 | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-24 | Fluocinolone Acetonide
67-73-2
|
US $9.00-60.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-24 | Fluocinolone acetonide
67-73-2
|
US $0.00-0.00 / KG | 1KG | 99% | 5000 | Wuhan Haorong Biotechnology Co.,Ltd |
-

- Fluocinolone Acetonide
67-73-2
- US $20.00-10.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD
-

- Fluocinolone Acetonide
67-73-2
- US $9.00-60.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD
-

- Fluocinolone acetonide
67-73-2
- US $0.00-0.00 / KG
- 99%
- Wuhan Haorong Biotechnology Co.,Ltd
67-73-2(fluocinolone16,17-acetonide)Related Search:
1of4