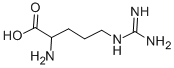
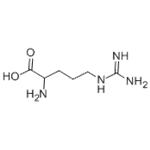
Arginine
- CAS No.
- 7004-12-8
- Chemical Name:
- Arginine
- Synonyms
- ARGININE;FMOC-L-ARG(PBF)-OPFP;FMOC-ARGININE(PBF)-OPFP;5-Guanidino-2-aminopentanoic acid;N-ALPHA-FMOC-N'-2,2,4,6,7-PENTAMETHYLDIHYDROBENZOFURAN-5-SULFONYL-L-ARGININE PENTAFLUORPHENYL ESTER;FMOC-N-OMEGA-(2,2,4,6,7-PENTAMETHYLDIHYDROBENZOFURAN-5-SULFONYL)-L-ARGININE PENTAFLUOROPHENYL ESTER;N-ALPHA-FMOC-NG-(2,2,4,6,7-PENTAMETHYLDIHYDROBENZOFURAN-5-SULFONYL)-L-ARGININE PENTAFLUOROPHENYL ESTER
- CBNumber:
- CB6467166
- Molecular Formula:
- C40H39F5N4O7S
- Molecular Weight:
- 814.82
- MDL Number:
- MFCD00237040
- MOL File:
- 7004-12-8.mol
| storage temp. | -15°C |
|---|---|
| pka | 1.82(at 25℃) |
| InChI | InChI=1S/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10) |
| InChIKey | ODKSFYDXXFIFQN-UHFFFAOYSA-N |
| SMILES | C(C(N)C(=O)O)CCNC(=N)N |
| FDA 21 CFR | 582.5145; 310.545 |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | arginine |
| FDA UNII | 94ZLA3W45F |
Arginine Chemical Properties,Uses,Production
Description
Arginine (abbreviated as Arg or R) is an α-amino acid. It was first isolated in 1886 . The L - form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that code for arginine during protein synthesis. In mammals, arginine is classified as a semiessential or conditionally essential amino acid, depending on the developmental stage and health status of the individual. Preterm infants are unable to synthesize or create arginine internally, making the amino acid nutritionally essential for them. There are some conditions that put an increased demand on the body for the synthesis of L-arginine, including surgical or other trauma, sepsis and burns . Arginine was first isolated from a lupin seedling extract in 1886 by the Swiss chemist Ernst Schultze.
In general, most people do not need to take arginine supplements because the body usually produces enough.
Chemical Properties
Prisms from water containing two molecules of H2O, anhydrous plates from alcohol solution. Dehydrates at 105C, decomposes at 244C. Sparingly soluble in alcohol; insoluble in ether.
Physical properties
The amino acid side-chain of arginine consists of a 3-carbon aliphatic straight chain, the distal end of which is capped by a complex guanidinium group.
With a pKa of 12.48, the guanidinium group is positively charged in neutral, acidic and even most basic environments, and thus imparts basic chemical properties to arginine. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized, enabling the formation of multiple H-bonds.
Occurrence
Dietary sources
Arginine is a conditionally nonessential amino acid, meaning most of the time it can be manufactured by the human body, and does not need to be obtained directly through the diet. The biosynthetic pathway however does not produce sufficient arginine, and some must still be consumed through diet. Individuals who have poor nutrition or certain physical conditions may be advised to increase their intake of foods containing arginine. Arginine is found in a wide variety of foods, including :
Animal sources
dairy products (e.g., cottage cheese, ricotta, milk, yogurt, whey protein drinks), beef, pork (e.g., bacon, ham), gelatin , poultry (e.g.chicken and turkey light meat), wild game (e.g. pheasant, quail), seafood (e.g., halibut, lobster, salmon, shrimp, snails, tuna)
Plant sources
wheat germ and flour, buckwheat, granola, oatmeal, peanuts, nuts (coconut, pecans, cashews, walnuts, almonds, Brazil nuts, hazelnuts, pinenuts), seeds (pumpkin, sesame, sunflower), chickpeas, cooked soybeans, Phalaris canariensis (canaryseed or ALPISTE)
Biosynthesis
Arginine is synthesized from citrulline by the sequential action of the cytosolic enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). In terms of energy, this is costly, as the synthesis of each molecule of argininosuccinate requires hydrolysis of adenosine tri phosphate (ATP) to adenosine mono phosphate (AMP), i.e., two ATP equivalents.
On a whole-body basis, synthesis of arginine occurs principally via the intestinal–renal axis, wherein epithelial cells of the small intestine, which produce citrulline primarily from glutamine and glutamate, collaborate with the proximal tubule cells of the kidney, which extract citrulline from the circulation and convert it to arginine, which is returned to the circulation. As a consequence, impairment of small bowel or renal function can reduce endogenous arginine synthesis, thereby increasing the dietary requirement.
Uses
Lung inflammation and asthma
Inhalation of L-arginine can increase lung inflammation and worsen asthma.
Growth hormone
Intravenously-administered arginine stimulates the secretion of growth hormone , and is used in growth hormone stimulation tests. Two studies have found that oral L-arginine supplementation is also effective at increasing resting GH levels. The first study found that oral preparations of L-arginine are effective at increasing growth hormone levels. In fact, the 9g dose resulted in mean peak GH levels of 6.4 (+/- 1.3) microg / L versus placebo levels of 2.9 (+/- 0.7).
MELAS syndrome
Several trials delved into effects of L-arginine in MELAS syndrome, a mitochondrial disease.
Sepsis
Cellular arginine biosynthetic capacity determined by activity of argininosuccinate synthetase (AS) is induced by the same mediators of septic response — endotoxin and cytokines — that induce nitric oxide synthase (NOS), the enzyme responsible for nitric oxide synthesis.
Malate salt
The malate salt of arginine can also be used during the treatment of alcoholic hepatitis and advanced cirrhosis.
Pre-eclampsia
A preliminary study of supplementation with L-arginine and antioxidant vitamins showed that this combination may help to combat abnormally high blood pressure during high risk pregnancies.
Hypertension
Intravenous infusion of arginine reduces blood pressure in patients with hypertension as well as normal subjects.A recent meta-analysis showed that L-arginine reduces blood pressure with pooled estimates of 5.4 / 2.7 mmHg for SBP / DBP.
Erectile dysfunction
Arginine taken in combination with proanthocyanidins or yohimbine, has also been used as a treatment for erectile dysfunction.
Anxiety
Dietary supplementation of L-arginine taken in combination with L-lysine has been shown potentially useful in treating people subjected to high levels of mental stress and anxiety, in a doubleblind, placebo controlled and randomized study, involving 108 Japanese adults. Trait anxiety and state anxiety induced by cognitive stress battery was significantly reduced, and basal levels of the stress hormone cortisol were decreased. The study was funded by Ajinomoto, Co. Inc., an industrial manufacturer of lysine and arginine.
Uses
Ammonia detoxicant; diagnostic aid (pituitary function determination).
Uses
Arginine is a nonessential amino acid that exists as white crystals or powder. it is soluble in water. it is used to improve the biological quality of the total protein in a food which contains naturally occur- ring primarily intact proteins and as a nutrient and dietary supplement.
Uses
arginine is also known as l-arginine. This is an amino acid with antistatic, masking, hair-and skin-conditioning properties.
Definition
An essential amino acid for rats, occurring naturally in the l(+) form. Available as glutamate and hydrochloride.
Biological Functions
Arginine plays an important role in cell division, the healing of wounds, removing ammonia from the body, immune function, and the release of hormones.
The benefits and functions attributed to oral supplementation of L-arginine include:
Precursor for the synthesis of nitric oxide (NO)
Reduces healing time of injuries (particularly bone)
Quickens repair time of damaged tissue
Helps decrease blood pressure in clinical hypertensive subjects.
Synthesis Reference(s)
Tetrahedron, 44, p. 805, 1988 DOI: 10.1016/0040-4039(96)01716-9
Tetrahedron Letters, 37, p. 7557, 1996 DOI: 10.1016/0040-4039(96)01716-9
Chemical and Pharmaceutical Bulletin, 29, p. 2825, 1981 DOI: 10.1248/cpb.29.2825
Arginine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 338 | 58 |
| GL Biochem (Shanghai) Ltd | 21-61263452 13641803416 | ymbetter@glbiochem.com | China | 9981 | 64 |
| Xibao Biotechnology (Shanghai) Co., Ltd. | 13761246140 | 58642714@qq.com | China | 415 | 58 |
| Nanjing Shizhou Biology Technology Co.,Ltd | 13675144456 | sean.lv@synzest.com | China | 10787 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 029-81124267 15229202216 | 1073@dideu.com | China | 10011 | 58 |
| Cato Research Chemicals Inc. | 020-81960175-611 18813968010 | xiang.liang@cato-chem.com | China | 904 | 58 |
| Anyang Hongmeng New Materials Co., Ltd | 0372-19588588067 19588588067 | 19588588067@163.com | China | 9332 | 58 |
Related articles
- What foods contain arginine in your daily diet?
- Arginine, which plays a vital role in life, has many sources and can be taken from the daily diet.
- Dec 25,2023