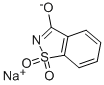
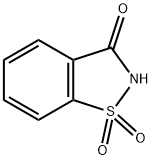
Saccharin sodium
- CAS No.
- 128-44-9
- Chemical Name:
- Saccharin sodium
- Synonyms
- SODIUM SACCHARIN;SACCHARIN SODIUM SALT;SACCHARINE SODIUM;204-886-1;o-sulfonbenzoicacidimidesodiumsalt;sucra;Saccharin sodium Anhydrous;dagutan;madhurin;willosetten
- CBNumber:
- CB6485865
- Molecular Formula:
- C7H5NNaO3S
- Molecular Weight:
- 206.17
- MDL Number:
- MFCD00013092
- MOL File:
- 128-44-9.mol
- MSDS File:
- SDS
| Melting point | >300°C |
|---|---|
| Density | 1.69[at 20℃] |
| vapor pressure | 0Pa at 25℃ |
| FEMA | 2997 | SACCHARINE, SODIUM SALT |
| storage temp. | 0-6°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless; sparingly soluble in ethanol. |
| pka | 0.003-0.003[at 20 ℃] |
| color | White crystals or a white, crystalline efflorescent powder |
| Odor | odourless or with a faint, aromatic odour |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | WINXNKPZLFISPD-UHFFFAOYSA-M |
| LogP | -2.84-0.11 at 25℃ |
| Substances Added to Food (formerly EAFUS) | SACCHARIN, SODIUM SALT |
| CAS DataBase Reference | 128-44-9(CAS DataBase Reference) |
| FDA UNII | I4807BK602 |
| EPA Substance Registry System | Saccharin sodium (128-44-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H312-H315-H319-H332-H335-H350 |
| Precautionary statements | P261-P280-P305+P351+P338 |
| Safety Statements | 24/25 |
| WGK Germany | 2 |
| RTECS | DE4550000 |
| Toxicity | LD50 oral in rat: 14200mg/kg |
Saccharin sodium price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S0050000 | Saccharin sodium European Pharmacopoeia (EP) Reference Standard | 128-44-9 | s0050000 | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR1348 | Saccharin Sodium | 128-44-9 | 500mg | $94.3 | 2024-03-01 | Buy |
| Matrix Scientific | 097940 | Sodium 3-oxo-3H-benzo[d]isothiazol-2-ide 1,1-dioxide 95+% | 128-44-9 | 5g | $45 | 2021-12-16 | Buy |
| Matrix Scientific | 097940 | Sodium 3-oxo-3H-benzo[d]isothiazol-2-ide 1,1-dioxide 95+% | 128-44-9 | 100g | $288 | 2021-12-16 | Buy |
| AK Scientific | Z6187 | Sodiumsaccharine | 128-44-9 | 5g | $110 | 2021-12-16 | Buy |
Saccharin sodium Chemical Properties,Uses,Production
Description
Saccharin sodium is a type of artificial or nonnutritive sweetener. It is 200 to 700 times sweeter than sucrose but has a bitter aftertaste. Saccharin and its salts do not occur naturally. Saccharin sodium is used in the production of various foods and pharmaceutical products including soft drinks, candy, biscuits, jams, chewing gum, tinned fruit, medicine and toothpaste.
Chemical Properties
Saccharin is a crystalline solid with a sweet taste (500 times sweeter than sugar). It is commercially available in three forms : acid saccharin , sodium saccharin , and calcium saccharin . These forms have been variously determined to be 200-800 times sweeter than sucrose , depending on the saccharin concentration. Saccharine sodium salt has no odor, but has an intensly sweet taste. Aqueous solution is neutral or alkaline to litmus, but not alkaline to phenolphthalein. Effloresces in dry air.

Sodium saccharin is widely used in food as a sweetener. There is evidence that sweet taste receptors of animals (pigs and bovines) also respond to saccharin (Hellekant et al., 1994; Moran et al., 2010).
Sodium saccharin is intended to be used in feed, premixtures and water for drinking for piglets (suckling and weaned piglets), pigs for fattening and calves for rearing up to 4 months and for calves for fattening up to 6 months, with levels up to 150 mg/kg of complete feedingstuffs and water for drinking.
Saccharin
Saccharin is the oldest artificial sweetener, and was discovered in 1879. The compound is prepared through reacting methyl anthranilate with nitrous acid sulfur dioxide, chlorine, and ammonia. It is about 300 times sweeter than sucrose and is considered to be one of the most important and widely used sweeteners worldwide.
Saccharin is a water-soluble acid with a pKa of 1.8. Its absorption is increased in animal species with lower stomach pH, such as rabbits and humans, relative to other mammals with higher stomach pHs such as rats. Other forms of saccharin that are consumed include: calcium saccharin, potassium saccharin, and acid saccharin. Sodium saccharin is used most often due to its greater palatability.
Saccharin, in addition to being used as a table-top sweetener, is commonly used in soft drinks, baked foods, jams, canned fruit, candy, dessert toppings, and chewing gum. Since saccharin’s sweetening power is not reduced when heated, it is an excellent candidate as an additive in low-calorie and sugar-free products.
Uses
Sodium saccharin is the salt most frequently used in formulating soluble forms of this sweetening agent. It can be used in toothpaste, mouthwash, diet soft drinks, syrups, baked goods, ice cream, and other sweet foods and drinks. While it is certainly most famously used in food products, sodium saccharin is also used in the chemical and agricultural industries as an aid in the production of herbicides and pesticides. It is also used as part of a solution used to coat metals, such as gold and nickel.
Major application of Sodium saccharine is the food industry as an additive in different products. It is used as a low calorie sweetener and stabilizer in a variety of food and drinks. In bakeries it is used to sweeten baked goods, breads, cookies and muffins. Due to its rapidly dissolving nature in water, it is used as an artificial sweetener in carbonated beverages and sodas.
Chemical Synthesis
Saccharin is chemically synthesised. The manufacturing process described by the applicant uses either phthalic anhydride or methyl anthranilate as starting material. Methyl anthranilate is diazotized to form 2-carbomethoxybenzene-diazonium chloride. Sulfonation followed by oxidation yields 2-carbomethoxybenzenesulfonyl chloride. Amidation of the sulfonylchloride followed by acidification will form insoluble acid saccharin. Subsequent addition of sodium hydroxide produces the soluble saccharin sodium.
Toxicity evaluation
Saccharin and toxicity are arguable. Throughout the 1960s, various studies suggested that saccharin might be an animal carcinogen [18]. Yilmaz and Uçar (2015) stressed that genotoxicity and carcinogenicity of saccharin are not understood clearly. Most publications reference that saccharin increases the rate of bladder cancer in rats fed with large doses.
A few epidemiological studies also found relationships between saccharin and bladder cancer risk in humans, but the majority of studies found no association between saccharin and cancer. Sodium saccharin has shown tumorigenic effects in rats. The compound was reported to produce hyperplastic response within a relatively short period of time when administered at high doses (≥2.5%).
Saccharin consumption has been associated with adverse effects on most of the biochemical and hematological blood indices in rats. Chronic saccharin intake affects biochemical parameters, and reported findings reflect various metabolic, hormonal, and neural responses in male and female rats resulting from the prolonged use of this sweetener after a single dose in drinking water. Consumption of large amounts of saccharin (135 mg) may result in hypoglycemia, reduced hyperinsulinemia, decreased insulin resistance, and improved glycemic control in hyperglycemic obese mice.
References
[1] DeeAn Jones, Environmental Fate of Cypermethrin
[2] http://www.inchem.org
[3] http://npic.orst.edu
Chemical Properties
Saccharin sodium occurs as a white, odorless or faintly aromatic, efflorescent, crystalline powder. It has an intensely sweet taste, with a metallic or bitter aftertaste that at normal levels of use can be detected by approximately 25% of the population. The aftertaste can be masked by blending saccharin sodium with other sweeteners. Saccharin sodium can contain variable amounts of water.
Uses
Saccharin Sodium is a flavoring agent and non-nutritive sweetener. It is a salt of saccharin widely used as sweetener in food and beverage. As a high-intensity sweetener, Saccharin Sodium can be used in a wide variety of industries including: food production, beverage, cosmetics, agriculture/animal feed, and various other industries. Saccharin Sodium Salt Hydrate can be used for the purification of recombinant polypeptides, such as antibodies.
Definition
ChEBI: Saccharin sodium is an organic molecular entity.
Production Methods
Saccharin is produced by the oxidation of o-toluene sulfonamide by potassium permanganate in a solution of sodium hydroxide. Acidification of the solution precipitates saccharin, which is then dissolved in water at 50℃ and neutralized by addition of sodium hydroxide. Rapid cooling of the solution initiates crystallization of saccharin sodium from the liquors.
brand name
Sucaryl (Ross).
General Description
Saccharin sodium appears as odorless white crystals or crystalline powder. Aqueous solution is neutral or alkaline to litmus, but not alkaline to phenolphthalein. Effloresces in dry air. Intensely sweet taste. (NTP, 1992)
Air & Water Reactions
Water soluble.
Reactivity Profile
Saccharin sodium may react with oxidizing agents. . Very weak base in aqueous solution.
Hazard
The use of saccharin is being limited due to possible carcinogenicity.
Fire Hazard
Flash point data are not available for Saccharin sodium , but Saccharin sodium is probably combustible.
Pharmaceutical Applications
Saccharin sodium is an intense sweetening agent used in beverages,
food products, table-top sweeteners, and pharmaceutical formulations
such as tablets, powders, medicated confectionery, gels,
suspensions, liquids, and mouthwashes. It is also used
in vitamin preparations.
Saccharin sodium is considerably more soluble in water than
saccharin, and is more frequently used in pharmaceutical formulations.
Its sweetening power is approximately 300–600 times that of
sucrose. Saccharin sodium enhances flavor systems and may be used
to mask some unpleasant taste characteristics.
Injection of saccharin sodium has been used to measure the armto-
tongue circulation time.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Moderately toxic by ingestion and intraperitoneal routes. A promoter. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of SOx, Na2O, and NOx.
Safety
There has been considerable controversy concerning the safety of
saccharin and saccharin sodium in recent years; however, it is now generally regarded as a safe, intense sweetener. See Saccharin for
further information.
The WHO has set a temporary acceptable daily intake of up to
2.5 mg/kg body-weight for saccharin, including its salts.(3) In the
UK, the Committee on Toxicity of Chemicals in Food, Consumer
Products, and the Environment (COT) has set an acceptable daily
intake for saccharin and its salts (expressed as saccharin sodium) at
up to 5 mg/kg body-weight.
LD50 (mouse, oral): 17.5 g/kg
LD50 (rat, IP): 7.1 g/kg
LD50 (rat, oral): 14.2 g/kg
Potential Exposure
The information provided has to do, primarily, with the manufacturing of saccharin. Saccharin has been used as a nonnutritive sweetening agent. At one point the United States consumption pattern for all forms of saccharin has been estimated as 45% in soft drinks; 18% in tabletop sweeteners; 14% in fruits, juices, sweets, chew- ing gum, and jellies; 10% in cosmetics and oral hygiene products; 7% in drugs, such as coating on pills; 2% in tobacco; 2% in electroplating; and 2% for miscellaneous uses. Human exposure to saccharin occurs primarily through ingestion because of its use in many dietic foods and drinks and some personal hygiene products, including toothpastes and mouthwashes. The general public is exposed to saccharin, especially by persons required to reduce sugar intake.
storage
Saccharin sodium is stable under the normal range of conditions
employed in formulations. Only when it is exposed to a high
temperature (125℃) at a low pH (pH 2) for over 1 hour does
significant decomposition occur. The 84% grade is the most stable
form of saccharin sodium since the 76% form will dry further under
ambient conditions. Solutions for injection can be sterilized by
autoclave.
Saccharin sodium should be stored in a well-closed container in a
dry place.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required.
Incompatibilities
Dust may form explosive mixture with air. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Incompatibilities
Saccharin sodium does not undergo Maillard browning.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contami- nant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.
Regulatory Status
Accepted for use as a food additive in Europe; ‘E954’ is applied to both saccharin and saccharin salts. Included in the FDA Inactive Ingredients Database (buccal and dental preparations; IM and IV injections; oral and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Saccharin sodium Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Pinestone Fortune International Trading Co., Ltd. | +86-027-85615902 +86-13971435335 | imp.exp8@fengfan.net | China | 29 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7534 | 58 |
| Shaanxi Franta Biotechnology Co., Ltd | +86-13082019107 +86-13082019107 | admin@flanderff.com | China | 229 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | Jany1001@kangcang.com.cn | China | 338 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 848 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
Related articles
- Use and preparation of Saccharin sodium
- Saccharin sodium is a kind of food additive and has no nutritional value to human body. When eating more, it will affect the n....
- Oct 14,2022
View Lastest Price from Saccharin sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | Saccharin sodium
128-44-9
|
US $9440.00-9430.00 / Tons | 1Tons | 99.99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-22 | Sodium Saccharin
128-44-9
|
US $9.00-80.00 / g | 10g | min99% | 10 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-22 | Saccharin sodium
128-44-9
|
US $20.00-15.00 / kg | 1000kg | 99% | 5000kg/week | Hebei Yime New Material Technology Co., Ltd. |
-

- Saccharin sodium
128-44-9
- US $9440.00-9430.00 / Tons
- 99.99%
- Hebei Dangtong Import and export Co LTD
-

- Sodium Saccharin
128-44-9
- US $9.00-80.00 / g
- min99%
- Hebei Kangcang new material Technology Co., LTD
-

- Saccharin sodium
128-44-9
- US $20.00-15.00 / kg
- 99%
- Hebei Yime New Material Technology Co., Ltd.
128-44-9(Saccharin sodium )Related Search:
1of4