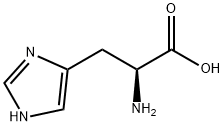
L-Histidine
- CAS No.
- 71-00-1
- Chemical Name:
- L-Histidine
- Synonyms
- HISTIDINE;HIS;H-HIS-OH;L-HIS;L-HISTIDINE BASE;L-His-OH;L-Histidin;(S)-2-aMino-3-(1H-iMidazol-4-yl)propanoic acid;Histidin;L-Hisidine
- CBNumber:
- CB6705020
- Molecular Formula:
- C6H9N3O2
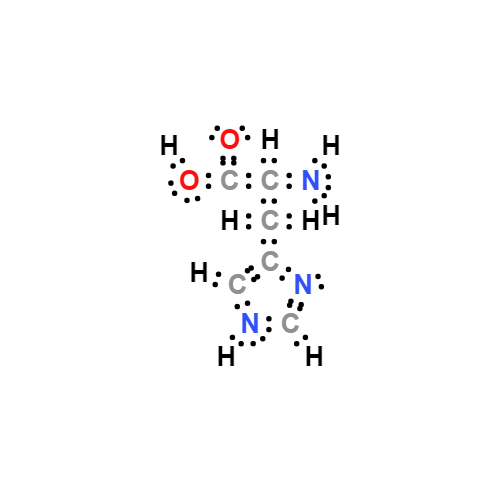
Lewis structure
- Molecular Weight:
- 155.15
- MDL Number:
- MFCD00064315
- MOL File:
- 71-00-1.mol
- MSDS File:
- SDS
| Melting point | 282 °C (dec.)(lit.) |
|---|---|
| alpha | 12.4 º (c=11,6N HCl) |
| Boiling point | 278.95°C (rough estimate) |
| Density | 1.3092 (rough estimate) |
| refractive index | 13 ° (C=11, 6mol/L HCl) |
| FEMA | 3694 | L-HISTIDINE |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| pka | 1.8(at 25℃) |
| color | White to off-white or pale yellow |
| PH | 7.0-8.0 (25℃, 0.1M in H2O) |
| PH Range | 7 - 8 at 15.5 g/l at 25 °C |
| Odor | odorless |
| Odor Type | odorless |
| optical activity | [α]20/D 39±1°, c = 2% in H2O |
| Water Solubility | 41.6 g/L (25 ºC) |
| λmax |
λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| JECFA Number | 1431 |
| Merck | 14,4720 |
| BRN | 4673585 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | HNDVDQJCIGZPNO-YFKPBYRVSA-N |
| LogP | -1.27 |
| Substances Added to Food (formerly EAFUS) | L-HISTIDINE |
| FDA 21 CFR | 172.320 |
| CAS DataBase Reference | 71-00-1(CAS DataBase Reference) |
| FDA UNII | 4QD397987E |
| NIST Chemistry Reference | L-Histidine(71-00-1) |
| EPA Substance Registry System | L-Histidine (71-00-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 24/25-36/37-26-22 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | MS3070000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29332990 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5110 mg/kg | |||||||||
| NFPA 704 |
|
L-Histidine price More Price(75)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 53319 | L-Histidine BioUltra, ≥99.5% (NT) | 71-00-1 | 25g | $69.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04352 | L-Histidine EMPROVE? EXPERT, Ph. Eur., ChP, JP, USP | 71-00-1 | 0.5KG | $631 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04352 | L-Histidine EMPROVE? EXPERT, Ph. Eur., ChP, JP, USP | 71-00-1 | 1KG | $1091 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1308505 | L-Histidine United States Pharmacopeia (USP) Reference Standard | 71-00-1 | 200mg | $466 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04351 | L-Histidine for biochemistry | 71-00-1 | 100g | $145 | 2024-03-01 | Buy |
L-Histidine Chemical Properties,Uses,Production
Chemical properties
White crystalline or crystalline powder,odorless,slightly bitter taste. Melting and decomposition at about 277~288 ℃. The imidazole and metal ions are easy to form complex salt. Dissolve in water (4.3g/100ml, 25 ℃), very difficult to dissolve in ethanol, insoluble in ether.
It is commonly used for its hydrochloride, because of minimal solubility and other reasons.
Uses
(1) nutritional supplements. It is the very important components of Amino acid infusion and comprehensive amino acid preparations. It can be used in the treatment of gastric ulcer, anemia, allergies and so on.
(2) It is used for biochemical research, medicine for the treatment of gastric ulcer, anemia, allergies and so on.
(3) It is used as amino acid drugs. It is the main components of amino acid infusion and amino acid preparations, for the treatment of gastric ulcer, anemia and angina, aortitis, heart failure and other cardiovascular system disorders.
Adverse reactions and contraindications: low toxicity, adult poisoning>64g/day, such as the injection of hydrochloric acid histidine with headache, flushing and heat.
(4) It is used as a nutrient enhancer, the important component of amino acid infusion and amino acid preparations. It can be used for the treatment of gastric ulcer and biochemical researchment.
(5) It is used for pharmaceutical raw materials and food additives.
Production methods
(1) It is eatracted from pig blood, bovine blood. Pig blood is spraied drying and then obtained blood powder, 100kg pig blood have 18kg blood power. L-histidine is commonly used as its hydrochloride salt ([7048-02-4]). The L-histidine containing eluent was concentrated to the appearance of crystals, adjusted to pH 2.5 with hydrochloric acid, and immediately added with 2 times the amount of ethanol in the solution, standing, precipitating and filtering to obtain L-group ammonia Acid hydrochloride crude, after decolorization, recrystallization, drying in the finished product. L-histidine can also be extracted from hydrolysates of defatted soybeans.
There are two main production methods. First one is direct fermentation, with carbon source of glucose and an inducible drug-resistant strain of corynebacterium glutamicum. Second one is protein hydrolysis. The hydrolysis method is described in detail below. Pig and cattle blood , pig hair or hoof were raw materials, hydrolyzed by acid , separated and purificated to get L-histidine.
Hydrolysis:50kg of pig blood powder and 4 times amout of 6mol/L HCl were put into a hydrolysis tank and heated at 110-120 ℃ for 24 hours.
Preparation of the dilution of the column: The hydrolysis solution of the previous step was concentrated under reduced pressure, distilled water was added again, and the acid was repeatedly distilled for 3-4 times until the distillate did not flow out of the hydrochloric acid. Concentrated solution diluted with distilled water to 500L,adjusted pH to 3.5-4 with concentrated ammonia, plused 20% of the amount of blood powder activated carbon, heated, decolorizated and stirred at 90 ℃ for 6h.Then filtered when it is hot, and take the filtrate standing overnight precipitate. Filtering again, the filtrate was diluted with distilled water to 2.5% (according to the blood powder dosage), and adjusted to pH 2.5 with concentrated HCl, which was column dilution.
The column dilution was separated, washed with water and eluted. Column is Ф300mm×2000 mm, PVC material, packed with 001 × 7 (732) strong acidic styrene cation exchange resin 1730mm, flow rate 1L/ min, stopping to upper column until the outflow of L histidine. Washing with water 500L, flow rate 1.5L /min. The pH 7.0-10.0 fraction was collected. After the collection, the resin was recoated for 15 min, and then regenerated with twice the amount of 1.5-2 mol/L HCl, the flow rate was 5-13 L/min. After regeneration, Washing with water untill PH 4 or so, waiting for the next column.
Purification: L-histidine acid eluent, removing ammonia with vacuum, concentrated to dry, and then dissolved with 40L distilled water, adjusted pH with 3-3.26 mol/L HCl, add 1kg activated carbon, heated and decolorizated at 90 ℃ for 30min.The filtrate was concentrated in a thin film evaporator and allowed to stand for 48 hours to precipitate crystals. The crystals were collected by filtration, washed with 95% ethanol and dried at 80 oC with vacuum for 4 hours to give L-histidine hydrochloride.
(2) Dry flour and hydrochloric acid as raw material reflux for several hours, filtrated,washed and handled with activated carbon to get L-histidine monohydrochloride crude, then purified to obtain the pure product.
(3) separated with ion exchange resin from the protein hydrolyzate of the basic amino acid.
Content analysis
Sample is accurately weighed about 105 rag, dried at 105 ℃ for 3h, then dissolved in 3ml formic acid and 50ml glacial acetic acid, and titrated with 0.1mol/L perchloric acid, and the end point was determined by potentiometric method. At the same time a blank test and the necessary amendments should be made. Per Ml0.1mol/L perchloric acid equates to 15.52mg L-histidine (C6H9N3O2).
Toxicity
It is safe for using in food products (FDA § 172.320, 2000).
Using limits
Accounting for 2.4% of total protein in food (FDA § 172.320, 2000).
FEMA: bakery products, meat products, dairy products, candy, frosting, are 150mg/kg.
Description
White, odorless crystals or crystalline powder having a slightly bitter taste. It is soluble in water, very slightly soluble in alcohol, and insoluble in ether. It melts with decomposition between about 277° and 288°C.
Chemical Properties
White or almost white, crystalline powder or colourless crystals
Chemical Properties
L-Histidine is an odorless powder with slightly bitter taste
Occurrence
Reported found in water bread, macaroni, egg noodles, corn flakes, corn grits, oatmeal, wheat bran, wheat flakes, shredded wheat, barley, brown rice, rye flour, whole grain wheat flour, buttermilk, blue cheese, cheddar cheese, cottage cheese, cream cheese, Parmesan cheese, bacon, cured ham, frankfurters, pork sausage, canned red kidney beans, canned sweet corn, canned peas, canned lima beans, canned potatoes, almonds, cashews, peanuts, dates, beef, lamb, veal, chicken, turkey and other natural sources
Uses
L-Histidine acts as a precursor to histamine and a component of carnosine. It is also used in medicine, feed additive, biochemical research, dietary supplement. It is used in a nutrition enhancer, is the amino acid fluid infusion. It can be used in the treatment of gastric ulcer.
Uses
L-Histidine has been used for characterization of ZnO nanorod in an attempt to develop a technique for the ultra-low level detection of l-histidine.
Uses
L-Histidine is an essential amino acid for human development which the body cannot produce on its own. L-Histidine is one of the 22 proteinogenic amino acids. L-Histidine precursor to histamine (H4365 00) and a component of carnosine.
Definition
ChEBI: The L-enantiomer of the amino acid histidine.
Biotechnological Production
L-Histidine is grouped with the aromatic amino acids, but the metabolic route diverges at an early stage from the other members of the group. It is produced in C. glutamicum in a 10-step sequence starting from phosphoribosyl pyrophosphate. Originally production titers of up to 10.5 g/L were reported, but this has since been increased by workers at Kyowa Hakko to 22.5 g/L. In a parallel development, the fermentation of L-histidine using E. coli has been reported by Ajinomoto, with titers up to 19.1 g/L. Both the titer and the carbon yield for L-histidine are lower than those reported for L-phenylalanine and L-tryptophan, and L-histidine remains one of the more challenging amino acids to produce on an industrial scale.
Biochem/physiol Actions
Precursor of histamine by action of histidine decarboxylase.
Purification Methods
A likely impurity is arginine. S-Histidine is adsorbed from aqueous solution onto a Dowex 50-H+ ion-exchange resin, washed with 1.5M HCl (to remove other amino acids), then eluted with 4M HCl as the dihydrochloride. This purified dihydrochloride (see below) is finally dissolved in water, the pH adjusted to 7.0, and the free zwitterionic base crystallises out on addition of EtOH. Its solubility in H2O is 4.2% at 25o. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 1971-1993 1961, Beilstein 25 III/IV 4344.]
L-Histidine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1100 | 58 |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8618980456393 | daisy@enlaibio.com | China | 1369 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 343 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
Related articles
- The benefits, uses and side effects of L-Histidine
- L-Histidine is an essential amino acid used in the production of proteins and is frequently present at the active site of prot....
- Nov 24,2023
- Health Benefits of L-Histidine
- L-histidine is a conditionally essential amino acid, so-called because adults generally produce adequate amounts of the substa....
- Oct 24,2019
View Lastest Price from L-Histidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | L-Histidine
71-00-1
|
US $5.00 / kg | 1kg | ≥98% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-18 | L-Histidine
71-00-1
|
US $50.00 / kg | 1kg | 99.10% | 50000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-18 | L-Histidine
71-00-1
|
US $30.00 / kg | 1kg | 99.7% | 200000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd |
-

- L-Histidine
71-00-1
- US $5.00 / kg
- ≥98%
- Jinan Finer Chemical Co., Ltd
-

- L-Histidine
71-00-1
- US $50.00 / kg
- 99.10%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- L-Histidine
71-00-1
- US $30.00 / kg
- 99.7%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
71-00-1(L-Histidine)Related Search:
1of4