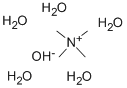
Tetramethylammonium hydroxide pentahydrate
- CAS No.
- 10424-65-4
- Chemical Name:
- Tetramethylammonium hydroxide pentahydrate
- Synonyms
- etramethylammonium Hydroxide Pentahydrate;TMAH Pentahydrate;Tetramethyl ammoniumhydroxid pentahydrate;MYXKPFMQWULLOH-UHFFFAOYSA-M;etramethylammonium Hydroxide;TETRAMETHYLAMM HYDROXIDE 5HYD;methanaminium,n,n,n-trimethyl;ammonium hydroxide pentahydrate;TETRAMETHYLAMMONIUM HYDROXIDE*PENTAH;Tetramethylammonium Hydroxide-D135 D2O
- CBNumber:
- CB7111710
- Molecular Formula:
- C4H23NO6
- Molecular Weight:
- 181.23
- MDL Number:
- MFCD00149566
- MOL File:
- 10424-65-4.mol
- MSDS File:
- SDS
| Melting point | 67-70 °C(lit.) |
|---|---|
| Density | 1.829 |
| vapor density | 1.1 (vs air) |
| vapor pressure | 97 mm Hg ( 20 °C) |
| storage temp. | 0-6°C |
| form | Hygroscopic Crystals |
| color | White |
| Odor | Ammonia like |
| explosive limit | 36% |
| Water Solubility | soluble |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,9224 |
| BRN | 3714235 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. |
| InChIKey | MYXKPFMQWULLOH-UHFFFAOYSA-M |
| CAS DataBase Reference | 10424-65-4(CAS DataBase Reference) |
| FDA UNII | B2HKT2LCKQ |
| EPA Substance Registry System | Methanaminium, N,N,N-trimethyl-, hydroxide, pentahydrate (10424-65-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310-H314-H370-H372-H411 | |||||||||
| Precautionary statements | P260-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T,C | |||||||||
| Risk Statements | 24/25-34-44-20/21/22 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 3423 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | PA0875000 | |||||||||
| F | 3-34 | |||||||||
| Autoignition Temperature | 878 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29239000 | |||||||||
| NFPA 704 |
|
Tetramethylammonium hydroxide pentahydrate price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 87741 | Tetramethylammonium hydroxide pentahydrate ≥95.0% (T) | 10424-65-4 | 25g | $74.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 87741 | Tetramethylammonium hydroxide pentahydrate ≥95.0% (T) | 10424-65-4 | 100g | $180 | 2024-03-01 | Buy |
| TCI Chemical | T1404 | Tetramethylammonium Hydroxide Pentahydrate >97.0%(T) | 10424-65-4 | 25g | $55 | 2024-03-01 | Buy |
| TCI Chemical | T1404 | Tetramethylammonium Hydroxide Pentahydrate >97.0%(T) | 10424-65-4 | 100g | $186 | 2024-03-01 | Buy |
| Alfa Aesar | L09658 | Tetramethylammonium hydroxide pentahydrate 98% | 10424-65-4 | 25g | $56.3 | 2024-03-01 | Buy |
Tetramethylammonium hydroxide pentahydrate Chemical Properties,Uses,Production
Outline
Tetramethylammonium hydroxide (TMAH) is the strongest organic base, it is colorless, odorless. Vapor pressure is low at room temperature, it can completely decompose and vaporize at 135~145℃, high purity product has no trace residue by low temperature treatment at 140℃.
Tetramethylammonium hydroxide solution is colorless and transparent with micro smell of ammonia, PH value of 1(wt)% solution is 12.9, it is strong alkali which has equal strength with caustic alkali. The tetramethylammonium hydroxide aqueous solution is concentrated under reduced pressure to obtain five crystal water absorbent needle-like crystals (mp, 63℃), then it continues dewatering into three crystal water of crystallization (mp, 63℃), then it turns into a crystal water of tetramethylammonium hydroxide, at 135~140℃, it can decompose into trimethylamine and dimethyl ether.
Uses
Tetramethylammonium hydroxide is organic base, it has very wide range of uses in the field of industrial research.
Domestic tetramethylammonium hydroxide is mainly used as organic silicon products, such as the catalyst of synthesizing silicone oil, silicone rubber, silicone resin, although the amount is not big, but the yield and quality of the product is greatly affected.
Foreign tetramethylammonium hydroxide is mainly used for polyester-based polymers, textiles, plastics, food, leather, wood processing, electroplating, and other microorganisms.
At present, this product has came into the advanced technology areas, such as in printed circuit board manufacturing and microscopic sheets, it can be used as cleaning agent in integrated circuit and anisotropic etchant in semiconductor microfabrication technology of the Si-SiO2 interface. With the development of science and technology, the requirement of this type of chemical is increasing, the quality and quantity of tetramethylammonium hydroxide have put forward higher requirements.
It can be used as additive of silicone rubber, methyl silicone oil and polarographic analysis reagents. In terms of organosilicone, it can be used as catalyst of dimethyl silicone oil, phenylmethyl silicone oil, a silicone oil diffusion pump, solvent-free silicone molding compound, silicone resin, silicone rubber or the like. In the analysis, it can be used as polarography agent, in the purification of the product it can be used as non-gray base to precipitate many metal elements, it can be used as silicon computer face brightener, cleaning agents and the like engraved touch in the production of organic silicon. The advantage of tetramethylammonium hydroxide as catalyst is that after the reaction when heat, it can decompose into gas and disappear, and it does not remain in the product.
preparation methods
It has many preparation methods of tetramethylammonium hydroxide, silver oxide method is commonly used, it is producted by the reaction of tetramethylammonium chloride with silver oxide. However, the preparation method of tetramethylammonium hydroxide is a complex process, the feedstock of silver oxide is expensive, and the resulting product contains higher impurity ions, such as halide ions, alkali metal ions, for the catalytic polymerization of organosilicon monomers, it has severe effect of silicone product features, and it can not meet the requirements for cleaning and corrosion in electronic fields.
From the seventies, foreign began to use new technology of electrolytic method to prepare tetramethylammonium hydroxide, and this method gradually replaced the silver oxide method. Electrolytic method has better product quality, low cost, it not only meets the needs of the production of organic silicon, but also has entered the electronics industry.
The above information is edited by the chemicalbook of Wang Xiaodong.
Process description of electrolysis
1. Tetramethylammonium chloride as raw material
The principle is that tetramethyl ammonium chloride aqueous solution in the electrolytic cell of anode compartment under the action of the electric force, the chloride ions in solution migrate to the anode and discharge of chlorine deposited on the anode. Meanwhile, since the ion-exchange membrane selective permeability, chloride diffusion can not go through the ion-exchange membrane, only tetramethylammonium ions can select and penetrate, then come into the cathode compartment, and enriched therein. The molecules water in electrolytic cell of cathode compartment can decompose into hydrogen and hydroxyl ions. The latter is exactly combined with tetramethylammonium ions from the anode chamber into tetramethylammonium hydroxide. With the increase of electricity, the concentration of tetramethylammonium hydroxide continues to improve to achieve the desired final concentration of crude.
Anodic electrochemical reaction is:
(CH3) 4NCl → (CH3) 4N ++ Clˉ
2Clˉ-2e → Cl2 ↑
Cathodic electrochemical reaction is:
H2O → H + + OHˉ
(CH3) 4N ++ OHˉ → (CH3) 4NOH
2H ++ 2e → H2 ↑
The overall reaction is: 2 (CH3) 4NCl + 2H2O → 2 (CH3) 4NOH + H2 ↑ + Cl2 ↑
The produced hydrogen of electrolysis is vented, resulting chlorine is absorbed with lye to generate sodium hypochlorite, sodium hypochlorite is the main raw material of bleach. Therefore, this preparation method of tetramethylammonium hydroxide is simple, high purity, and has no environmental pollution.
2.Tetramethyl-ammonium bicarbonate as starting material
The theory of electrolysis is similar with tetramethylammonium chloride as raw material, but chlorine can not generate in this method, carbon dioxide and oxygen generate at the anode. Total reaction: (CH3) 4NHCO3 + H2O (CH3) 4NOH + CO2 ↑ + H2 ↑
Chemical properties
It is colorless crystal (often with three, five crystal water, etc.), it can easily absorb moisture in the air can rapidly absorb carbon dioxide, it can decompose into methanol and trimethylamine at 130℃, it usually has 10%, 25% water (or alcohol) aqueous solution and containing crystal compound.
Chemical Properties
moist white crystals with an amine odour
Uses
Tetramethylammonium hydroxide pentahydrate is a clathrate hydrate, which shows high proton conductivity. It can readily uptake carbon dioxide. It is a promising candidate for energy devices, gas separation, and gas storage applications.
Tetramethylammonium hydroxide pentahydrate may be used in the preparation of a mesoporous molecular sieve belonging to the MCM-41 (mobil composition of matter No. 41) category with silica to (tetrahedral) aluminum ratio as low as 8.5:1.
Uses
Tetramethylammonium Hydroxide Pentahydrate is a useful compound for preparing gamma-cesium lead iodide films for high-efficiency deep-red light-emitting devices.
Uses
Tetramethylammonium hydroxide pentahydrate has been used in a study to assess strains of Pseudomnas species by a single-step gas chromatographic characterization procedure. It has also been used in a study to investigate solid-state metal hydride batteries nickel oxyhydroxide-metal hydride and manganese dioxide-metal hydride.
General Description
Tetramethylammonium hydroxide pentahydrate is a clathrate hydrate, which shows high proton conductivity. It can readily uptake carbon dioxide. It is a promising candidate for energy devices, gas separation, and gas storage applications.
Purification Methods
It is freed from chloride ions by passage through an ion-exchange column (e.g. Amberlite IRA-400, prepared in its OH-form by passing 2M NaOH until the effluent is free from chloride ions, then washed with distilled H2O until neutral). A modification, to obtain carbonate-free hydroxide, uses the method of Davies and Nancollas [Nature 165 237 1950]. [Beilstein 4 IV 145.]
Tetramethylammonium hydroxide pentahydrate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jinan Qinmu Fine Chemical Co.,Ltd. | +8618660799346 | sales@qinmuchem.com | China | 1009 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
View Lastest Price from Tetramethylammonium hydroxide pentahydrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-14 | Tetramethylammonium hydroxide pentahydrate
10424-65-4
|
US $0.00 / kg | 1kg | 0.99 | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-09-06 | Tetramethylammonium hydroxide pentahydrate
10424-65-4
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-08-02 | Tetramethylammonium hydroxide pentahydrate
10424-65-4
|
US $100.00 / KG | 1KG | 99% | 5000KG | Hebei Mojin Biotechnology Co., Ltd |
-

- Tetramethylammonium hydroxide pentahydrate
10424-65-4
- US $0.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- Tetramethylammonium hydroxide pentahydrate
10424-65-4
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- Tetramethylammonium hydroxide pentahydrate
10424-65-4
- US $100.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
10424-65-4(Tetramethylammonium hydroxide pentahydrate)Related Search:
1of4