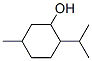
MENTHOL
- CAS No.
- 15356-70-4
- Chemical Name:
- MENTHOL
- Synonyms
- D-Menthol;Menthol racemic;2-Isopropyl-5-methylcyclohexanol;racementhol;FEMA 2665;MENTHOL;NCI-C50000;3-p-Menthol;d,1-menthol;MENTHOL, DL-
- CBNumber:
- CB7116412
- Molecular Formula:
- C10H20O
- Molecular Weight:
- 156.27
- MDL Number:
- MFCD00001484
- MOL File:
- 15356-70-4.mol
- MSDS File:
- SDS
| Melting point | 34-36 °C(lit.) |
|---|---|
| Boiling point | 216 °C(lit.) |
| Density | 0.89 g/mL at 25 °C(lit.) |
| vapor pressure | 0.8 mm Hg ( 20 °C) |
| FEMA | 2665 | MENTHOL RACEMIC |
| refractive index | 1.4615 |
| Flash point | 200 °F |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, very soluble in ethanol (96 per cent) and in light petroleum, freely soluble in fatty oils and in liquid paraffin, very slightly soluble in glycerol. |
| form | cryst. |
| color | White |
| Dielectric constant | 3.2(Ambient) |
| InChIKey | NOOLISFMXDJSKH-UHFFFAOYSA-N |
| CAS DataBase Reference | 15356-70-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | YS08XHA860 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H315-H319-H331-H336-H351-H361d-H372 |
| Precautionary statements | P202-P301+P312-P302+P352-P304+P340+P311-P305+P351+P338-P308+P313 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 37/38-41-48/20/22-40-38-22 |
| Safety Statements | 36/37/39-36-26 |
| RIDADR | UN 1888 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | OT0525000 |
| HS Code | 29061100 |
MENTHOL price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | CRM40467 | D,L-Menthol solution certified reference material, 2000?μg/mL in methanol, ampule of 1?mL | 15356-70-4 | 1mL | $75 | 2024-03-01 | Buy |
| JR MediChem | JR8-N0276 | DL-Menthol 98% | 15356-70-4 | 20mg | $100 | 2021-12-16 | Buy |
| Arctom | CFN99160 | DL-Menthol ≥98% | 15356-70-4 | 20mg | $30 | 2021-12-16 | Buy |
MENTHOL Chemical Properties,Uses,Production
Chemical Properties
Menthol has three asymmetric carbon atoms in its cyclohexane ring and, therefore, occurs as four pairs of optical isomers.The configurations of four of these isomers are given; the other four are their mirror images.(1R,3R,4S)-(?)-Menthol,,; (1R,3S,4S)-(+)-neomenthol,; (1R,3S,4R)-(+)- isomenthol,; (1R,3R,4R)-(+)-neoisomenthol,. (?)-Menthol is the isomer that occurs most widely in nature. It is the main component of peppermint and cornmint oils obtained from the Mentha piperita and Mentha arvensis species. Esterified menthol also occurs in these oils (e.g., as the acetate and isovalerate).Other menthol stereoisomers may be present in these oils as well.
Chemical Properties
Racemic menthol is a mixture of equal parts of the (1R,2S,5R)- and (1S,2R,5S)-isomers of menthol. It is a free-flowing or agglomerated crystalline powder, or colorless, prismatic, or acicular shiny crystals, or hexagonal or fused masses with a strong characteristic odor and taste. The crystalline form may change with time owing to sublimation within a closed vessel. The USP 32 specifies that menthol may be either naturally occurring l-menthol or syntheti-cally prepared racemic or dl-menthol. However, the JP XV and PhEur 6.0, along with other pharmacopeias, include two separate monographs for racemic and l-menthol.
Chemical Properties
Free-flowing or agglomerated, crystalline powder or prismatic or acicular, colourless, shiny crystals
Physical properties
Physical Properties. The eight optically active menthols differ in their sensory
properties. (?)-Menthol has a characteristic peppermint odor and also
exerts a cooling effect. The other isomers do not possess this cooling effect and
are, therefore, not considered to be “refreshing.” Racemic menthol occupies an
intermediate position; the cooling effect of the (?)-menthol present is distinctly
perceptible.
The enantiomeric menthols have identical physical properties (apart from their
specific rotations), but the racemates differ from the optically active forms in, for
example, theirmelting points.Although the differences between the boiling points
are small, the (racemic) stereoisomers can be separated by fractional distillation.
Boiling points (in °C at 101.3 kPa) are as follows:
rac-neomenthol, 211.7
rac-neoisomenthol 214.6
rac-menthol, 216.5
rac-isomenthol, 218.6
Other physical constants of commercially available levorotatory and racemic
menthols are as follows: (?)-menthol, mp 43 °C, [α]20
D ?50° (ethanol, 10%);
racemic menthol, mp 38 °C.
Chemical Properties. Hydrogenation of menthols yields p-menthane; oxidation
with chromic acid or catalytic dehydrogenation yields menthones. Dehydration
under mild conditions yields 3-p-menthene as the main product. Reaction with
carboxylic acids or their derivatives yields menthyl esters, which are used mainly
as aroma substances and in pharmaceutical preparations and formulations. The
esterification of menthols with benzoic acid is used on an industrial scale in the
resolution of racemic menthol.
Occurrence
Has apparently not been reported to occur in nature
Preparation
By hydrogenation of thymol followed by separation from its other isomers.
Indications
Menthol, a cyclic alcohol (derived from peppermint, other mint oils, or prepared synthetically), relieves itching by generating a cool sensation. It is usually used in 0.25% to 2% concentrations but is present in concentrations as high as 16% in some OTC products.
Production Methods
Menthol occurs widely in nature as l-menthol and is the principal
component of peppermint and cornmint oils obtained from the
Mentha piperita and Mentha arvensis species. Commercially, lmenthol
is mainly produced by extraction from these volatile oils. It
may also be prepared by partial or total synthetic methods.
Racemic menthol is prepared synthetically via a number of
routes, e.g. by hydrogenation of thymol.
Definition
ChEBI: P-menthan-3-ol is any secondary alcohol that is one of the eight possible diastereoisomers of 5-methyl-2-(propan-2-yl)cyclohexan-1-ol. It has a role as a volatile oil component. It is a p-menthane monoterpenoid and a secondary alcohol.
Toxicity evaluation
The acute oral LD50 in rats has been reported as 3180 mg/kg by Jenner, Hagan, Taylor, Cook & Fitzhugh (1964) and as 2900 mg/kg by Herken (1961). The acute oral LD50 in cats was reported to be 1500-1600 mg/kg (Flury & Seel, 1926). The sc LD50 in the mouse was reported as 1400-1600 mg/kg (Flury & Seel, 1926) and the ip LD50 as 750 mg/kg in the rat (Herken, 1961) and 1500-1600 mg/kg in the cat (Flury & Seel, 1926). In rabbits, the ip LD50 was reported to be approximately 2000 mg/kg (Herken, 1961), while the acute dermal LD50 exceeded 5000 mg/kg (Levenstein, 1973).
Pharmaceutical Applications
Menthol is widely used in pharmaceuticals, confectionery, and
toiletry products as a flavoring agent or odor enhancer. In addition
to its characteristic peppermint flavor, l-menthol, which occurs
naturally, also exerts a cooling or refreshing sensation that is
exploited in many topical preparations. Unlike mannitol, which
exerts a similar effect due to a negative heat of solution, l-menthol
interacts directly with the body’s coldness receptors. d-Menthol has
no cooling effect, while racemic menthol exerts an effect approximately
half that of l-menthol.
When used to flavor tablets, menthol is generally dissolved in
ethanol (95%) and sprayed onto tablet granules and not used as a
solid excipient.
Menthol has been investigated as a skin-penetration enhancer
and is also used in perfumery, tobacco products, chewing gum and
as a therapeutic agent. When applied to the skin, menthol dilates the
blood vessels, causing a sensation of coldness followed by an
analgesic effect. It relieves itching and is used in creams, lotions, and
ointments. When administered orally in small doses menthol has a
carminative action.
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. An eye and skin irritant. K%en heated to decomposition it emits acrid smoke and irritating fumes. See also MENTHOL and 1-MENTHOL.
Safety
Almost all toxicological data for menthol relate to its use as a
therapeutic agent rather than as an excipient. Inhalation or
ingestion of large quantities can result in serious adverse reactions
such as ataxia and CNS depression,hypersensitivity reactions,
severe abdominal pain, nausea, vomiting, vertigo, drowsiness, and
coma.Although menthol is essentially nonirritant there have been
some reports of hypersensitivity following topical application.
In a Polish study approximately 1% of individuals were determined
as being sensitive to menthol.There have been reports of apnea
and instant collapse in infants after the local application of menthol
to their nostrils.
The WHO has set an acceptable daily intake of menthol at up to
0.4 mg/kg body-weight.
LD50 (rat, IM): 10.0 g/kg
LD50 (rat, oral): 3.18 g/kg
target
GABAA receptor
Metabolism
Rabbits are said to eliminate 59% of dl-menthol as glucuronide (Williams, 1938).
storage
A formulation containing menthol 1% w/w in aqueous cream has
been reported to be stable for up to 18 months when stored at room
temperature.
Menthol should be stored in a well-closed container at a
temperature not exceeding 25°C, since it sublimes readily.
Incompatibilities
Incompatible with: butylchloral hydrate; camphor; chloral hydrate; chromium trioxide; b-naphthol; phenol; potassium permanganate; pyrogallol; resorcinol; and thymol.
Regulatory Status
Included in the FDA Inactive Ingredients Database (dental preparations, inhalations, oral aerosols, capsules, solutions, suspensions, syrups, and tablets; also topical preparations). Included in nonparenteral medicines licensed in the UK. Accepted for use in foods and confectionery as a flavoring agent of natural origin. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
MENTHOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from MENTHOL manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | DL-Menthol
15356-70-4
|
US $0.00 / kg | 1kg | ≥98% HPLC | 1000kg | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-03-28 | DL-Menthol
15356-70-4
|
US $0.00 / kg | 25kg | 98% | Inquiry | PNP Biotech Co. Ltd | |
 |
2024-03-11 | MENTHOL
15356-70-4
|
US $25.00-11.00 / kg | 500kg | 99.9 | 200tons | Shandong Juchuang Chemical Co., LTD |
-

- DL-Menthol
15356-70-4
- US $0.00 / kg
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- DL-Menthol
15356-70-4
- US $0.00 / kg
- 98%
- PNP Biotech Co. Ltd
-

- MENTHOL
15356-70-4
- US $25.00-11.00 / kg
- 99.9
- Shandong Juchuang Chemical Co., LTD
15356-70-4(MENTHOL)Related Search:
1of4