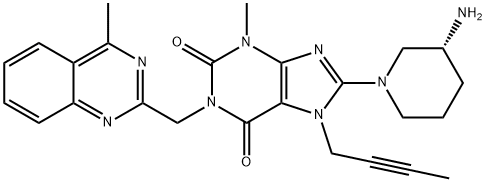
Linagliptin
- CAS No.
- 668270-12-0
- Chemical Name:
- Linagliptin
- Synonyms
- Linagliptin API;Linaglitpin;Linagliptin (BI-1356);8-[(3R)-3-Amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione;inag;iptin;inagL;Ondero;CS-494;Bi 1356
- CBNumber:
- CB71518518
- Molecular Formula:
- C25H28N8O2
- Molecular Weight:
- 472.54
- MDL Number:
- MFCD14635356
- MOL File:
- 668270-12-0.mol
- MSDS File:
- SDS
| Melting point | 202 ºC |
|---|---|
| Boiling point | 661.2±65.0 °C(Predicted) |
| Density | 1.39 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| pka | 10.01±0.20(Predicted) |
| form | Solid |
| color | White to Orange |
| InChIKey | LTXREWYXXSTFRX-QGZVFWFLSA-N |
| SMILES | N1(CC#CC)C2=C(N(C)C(=O)N(CC3=NC(C)=C4C(=N3)C=CC=C4)C2=O)N=C1N1CCC[C@@H](N)C1 |
| FDA UNII | 3X29ZEJ4R2 |
| ATC code | A10BH05 |
| NCI Drug Dictionary | linagliptin |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H335-H315 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 | |||||||||
| HS Code | 29335990 | |||||||||
| NFPA 704 |
|
Linagliptin price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 23306 | Linagliptin ≥98% | 668270-12-0 | 100mg | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 23306 | Linagliptin ≥98% | 668270-12-0 | 500mg | $120 | 2024-03-01 | Buy |
| Cayman Chemical | 23306 | Linagliptin ≥98% | 668270-12-0 | 1g | $225 | 2024-03-01 | Buy |
| TRC | L465900 | Linagliptin | 668270-12-0 | 500mg | $120 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | D47119 | Linagliptin-d3 | 668270-12-0 | 25mg | $5450 | 2021-12-16 | Buy |
Linagliptin Chemical Properties,Uses,Production
Uses
Linagliptin (TrajentaR, TradjentaTM, TrazentaTM, TrayentaTM) is an oral, highly selective inhibitor of dipeptidyl peptidase-4 and is the first agent of its class to be eliminated predominantly via a nonrenal route. Linagliptin is indicated for once daily use for the treatment of adults with type 2 diabetes mellitus.
Treatment of Type 2 diabetes
Linagliptin acts to lower blood glucose levels by inhibiting the enzyme DPP-4, thereby preventing the degradation of the incretin hormones (glucagon-like peptide-1 [GLP-1] and glucose-dependent insulinotropic peptide) and attenuating postprandial glucose excursions. By selectively targeting DPP-4, linagliptin potentially causes a more physiologically based control of glucose-dependent postprandial glucose excursions and of fasting blood glucose, both of which are mediated by effects of glucose on insulin and glucagon secretion. An advantage of linagliptin is that since incretin-stimulated release of insulin is glucose dependent, linagliptin is associated with a low incidence of hypoglycaemia. Moreover, DPP-4 inhibitors have a low potential for drug-drug interactions (with the exception of saxagliptin, which is metabolized by cytochrome P450 [CYP] 3A4/5), are generally well tolerated and have minimal or neutral effects on bodyweight.
Pharmacokinetics
Linagliptin shows modest oral bioavailability, and it is rapidly absorbed. The maximum plasma concentration at steady state is reached on average 1.5 hours after administration of linagliptin 5 mg, once daily . Linagliptin half-life is 131 hours. No relevant food effects were observed on the absorption profile of linagliptin. Unlike other DPP-4 inhibitors, linagliptin excretion is not performed by the kidneys, but rather through the enterohepatic system.
Description
Linagliptin (trade names Tradjenta and Trajetna) is an inhibitor of dipeptidyl peptidase-4 (DPP-4) that was approved by the U.S. FDA in May 2011 for the treatment of Type 2 diabetes along with diet and exercise. Linagliptin (BI-1356) has been described as a potent highly selective, slow-off rate and long acting inhibitor of DPP-4. Linagliptin arose from optimization efforts of xanthine-based DPP-4 inhibitors with the initial lead identified from an HTS campaign. After optimizing the activity of the initial micromolar lead, two issues that needed to be addressed were activity for hERG and muscarinic receptor M1. Introduction of a butynyl group at the N7 position of the xanthine ring gave much reduced M1 affinity with no measureable hERG activity. Linagliptin inhibits DPP-4 with an IC50=1 nM and is highly selective (>10,000-fold) against DPP-8 and DPP-9. Linagliptin shows no interactions with CYPs up to 50 mM. The described synthesis of linagliptin starts with 8-bromoxanthine, which is alkylated at the N-7 position to introduce the butyne group, followed by alkylation of the N-1 group to introduce the methyl-quinazoline group. Displacement of the bromide with (R)-Boc-3-amino-piperidine followed by deprotection gives linagliptin. When administered to db/db mice orally, linagliptin dose dependently reduced glucose excursion from 0.1 mg/kg (15% inhibition) to 1 mg/kg (66% inhibition).
Originator
Boehringer Ingelheim (United States)
Uses
dipeptidypeptidase inhibitor, antidiabetic
Uses
A novel potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor with potential use in the treatment of type 2 diabetes.
Uses
Labeled Linagliptin, intended for use as an internal standard for the quantification of Linagliptin by GC- or LC-mass spectrometry.
Uses
highly potent CD26 inhibitor
Definition
ChEBI: A xanthine that is 7H-xanthine bearing (4-methylquinazolin-2-yl)methyl, methyl, but-2-yn-1-yl and 3-aminopiperidin-1-yl substituents at positions 1, 3, 7 and 8 respectively (the R-enantiomer). Used for treatment of type I diabetes.
brand name
Tradjenta
Clinical Use
Type 2 diabetes mellitus
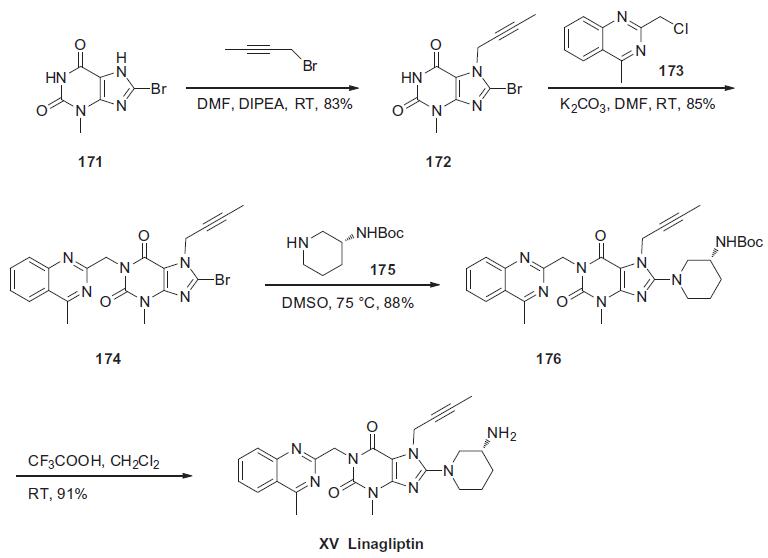
Synthesis
The synthesis of linagliptin began from commercially available 8-bromo-3-methylxanthine (171). Sequential alkylations of guanine derivative 171 at N-7 with butyn-2-yl bromide in the presence of N,N-diisopropylethylamine and N-1 with 2- (chloromethyl)-4-methylquinazoline (173) in the presence of potassium carbonate, yielded N1,N7-dialkylated xanthine 174 in 85% yield. This material was further condensed with (R)-3-Bocaminopiperidine (175) in the presence of potassium carbonate to give aminopurine dione 176 in 88% yield. Finally, the primary amine of 176 was liberated with trifluoroacetic acid in methylene chloride to produce linagliptin (XV) in 91% yield.
target
DDP-4
storage
+4°C
Linagliptin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ShenZhen H&D Pharmaceutical Technology Co., LTD | +8613627253706 | sale@hdimpurity.com | China | 1501 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7534 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 | sarah@tnjone.com | China | 848 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 885 | 50 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9350 | 55 |
Related articles
- What is Linagliptin?
- Linagliptin (TrajentaR, TradjentaTM, TrazentaTM, TrayentaTM) is an oral, highly selective inhibitor of dipeptidyl peptidase-4 ....
- Feb 10,2020
View Lastest Price from Linagliptin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Linagliptin
668270-12-0
|
US $20.00-5.00 / g/Bag | 100g/Bag | 99% up, High Density | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-13 | Linagliptin
668270-12-0
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-03-29 | Linagliptin
668270-12-0
|
US $0.00 / mg | 10mg | 0.98 | 10g | ShenZhen H&D Pharmaceutical Technology Co., LTD |
-

- Linagliptin
668270-12-0
- US $20.00-5.00 / g/Bag
- 99% up, High Density
- Sinoway Industrial co., ltd.
-

- Linagliptin
668270-12-0
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Linagliptin
668270-12-0
- US $0.00 / mg
- 0.98
- ShenZhen H&D Pharmaceutical Technology Co., LTD
668270-12-0(Linagliptin)Related Search:
1of4