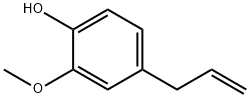
Eugenol
- CAS No.
- 97-53-0
- Chemical Name:
- Eugenol
- Synonyms
- 4-ALLYL-2-METHOXYPHENOL;Eug;Engenol;4-allyl-2-methoxyphenol (eugenol);p-Eugenol;FEMA 2467;Eugenol,99%;5-allylguaiacol;2-Methoxy-4-prop-2-enylphenol;2-METHOXY-4-(2-PROPENYL)PHENOL
- CBNumber:
- CB7208326
- Molecular Formula:
- C10H12O2
Lewis structure

- Molecular Weight:
- 164.2
- MDL Number:
- MFCD00008654
- MOL File:
- 97-53-0.mol
- MSDS File:
- SDS
| Melting point | -12--10 °C (lit.) |
|---|---|
| Boiling point | 254 °C (lit.) |
| Density | 1.067 g/mL at 25 °C (lit.) |
| vapor pressure | <0.1 hPa (25 °C) |
| FEMA | 2467 | EUGENOL |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| solubility | 2.46g/l |
| form | Liquid |
| pka | pKa 9.8 (Uncertain) |
| color | Clear pale yellow to yellow |
| Odor | at 10.00 % in dipropylene glycol. sweet spicy clove woody |
| Odor Type | spicy |
| Water Solubility | slightly soluble |
| Sensitive | Air Sensitive |
| Merck | 14,3898 |
| JECFA Number | 1529 |
| BRN | 1366759 |
| Dielectric constant | 6.1(18℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | RRAFCDWBNXTKKO-UHFFFAOYSA-N |
| LogP | 1.83 at 30℃ |
| Substances Added to Food (formerly EAFUS) | EUGENOL |
| FDA 21 CFR | 177.2800; 310.545; 582.60 |
| CAS DataBase Reference | 97-53-0(CAS DataBase Reference) |
| EWG's Food Scores | 4-7 |
| FDA UNII | 3T8H1794QW |
| NIST Chemistry Reference | Eugenol(97-53-0) |
| IARC | 3 (Vol. 36, Sup 7) 1987 |
| EPA Substance Registry System | Eugenol (97-53-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317-H319 | |||||||||
| Precautionary statements | P261-P264-P272-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-42/43-38-40-43-36/38 | |||||||||
| Safety Statements | 26-36-24/25-23-36/37 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | SJ4375000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29095090 | |||||||||
| Toxicity | LD50 in rats, mice (mg/kg): 2680, 3000 orally (Hagan) | |||||||||
| NFPA 704 |
|
Eugenol price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W246719 | Eugenol natural, ≥98%, FG | 97-53-0 | 1kg | $109 | 2024-03-01 | Buy |
| Sigma-Aldrich | W246719 | Eugenol natural, ≥98%, FG | 97-53-0 | 5kg | $599 | 2024-03-01 | Buy |
| Sigma-Aldrich | W246719 | Eugenol natural, ≥98%, FG | 97-53-0 | 10Kg | $940 | 2024-03-01 | Buy |
| Sigma-Aldrich | W246700 | Eugenol ≥98%, FCC, FG | 97-53-0 | 1kg | $125 | 2024-03-01 | Buy |
| Sigma-Aldrich | W246700 | Eugenol ≥98%, FCC, FG | 97-53-0 | 5kg | $442 | 2024-03-01 | Buy |
Eugenol Chemical Properties,Uses,Production
Flavor
Eugenol exists naturally in eugenia oil, basil oil and cinnamon oil and other essential oils. It is a thick oily liquid, colorless to pale yellow, with a strong aroma of clove and a pungent aroma.
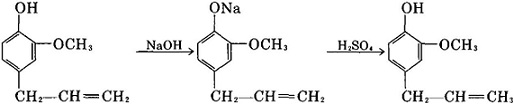
At present, most of the industries deal with essential oils rich in eugenol with alkali to produce eugenol. The sodium hydroxide solution is usually added to the separated oil, and then the mixture is heated and stirred. The oil of the non-phenol part floating on the liquid surface is extracted with a solvent, and it could be steamed off also. Add acid to acidify sodium salt to obtain crude eugenol, and then wash with water to neutral state, and finally distill in vacuum to get pure eugenol.
Physical and chemical properties
The scientific name for eugenol is 4-allyl-2-methoxyphenol. Some data about it are as follows: molecular formula C10H12O2; molecular weight 164.21; boiling point 253 ℃; melting point-9.2 ~-9.1℃; relative density d2525 1.053~1.064; refractive index nD20 1.538~1.542. It is miscible with alcohol, ether, chloroform and volatile oil. What’s more, it is slightly soluble in water, and soluble in acetic acid and caustic solution. It gradually darkens and thickens in the air. Iron, zinc and other metal ions could catalyze its oxidation. So we should store it below 25 ℃, and protect it from light. Eugenol could make the red litmus and ferric chloride ethanol solution blue. It is present in the clove oil (90%), clove basil oil (about 60%), violet flower oil (about 20%), cinnamon leaf oil, lauric oil, camphor oil, acacia oil and citronella oil. It can be used as fixative and modifier for woody and oriental essence in the spice. It is the main essence for preparation of clove and carnation flavor. It is also often used in the mint, nut and spicy food flavor and tobacco flavor. It can also be used to synthesize vanillin. It is applied for medical and health products and dental hygiene. Eugenol is available in the United States FEMA2467, and it is approved for food by US FDA.
Preparation
Guaiacol is used as raw material. Allyl chloride and allyl alcohol have a direct effect of allylation on guaiacol (patent in the former Soviet Union 352872; US patent 3929904).
There are some disadvantages of synthetic method in making eugenol. These shortcomings need to be improved in the future. Disadvantages are as follows: Many side effects result in difficult separation and purification, and they also affect the quality of product.
Eugenol is used in the formula of perfume essence, a variety of cosmetic essence and soap essence, with the amount of less than 20%. It can also be used in food flavors. There is no restriction in IFRA.

Lilac
Clove tree is a variant name for lilac. It is bud of Syzygium Myrtaceous plant. There are more than 500 kinds of Syzygium in the world, about 72 species in China.
Clove is a tropical and humid forest plant. The growth environment should be warm and humid, and should not be cold. Uniform and abundant rain is needed. In addition, deep, fertile and well-drained soil is better. It can be harvested when the buds turn from green to red in September to March of the following year. After it is harvested, we should remove the pedicel and dry it.
The bud is nail-shaped, and the length is 1~2cm. Calyx tube is cylindrical and slightly quadrangular, with the length of 6~14mm, diameter of about 5mm. The base gradually becomes narrow. And rough surface engraved seepage oil. The upper part has 4triangular sepals that are reddish brown or dark brown and the length is about 3mm. The top corolla is round, with diameter of 4 to 6 mm. 4 tan petals wrap around in tiles. Cut open buds, we could see the situation: a lot of stamens; filaments bent to the center; the central has a straight sturdy which is easy to sink in the water. The section is oily. It has a strong aroma and spicy taste, with a sense of hemp. Lilac is better with these characteristics: large, stout, fresh purple brown, strong aroma, more oil. There are volatile oil 16~19% in buds. The main oil contained in volatile oil are eugenol 80~87%, β-clove 9%, aceto-eugenol7%. In addition, some trace components are present, for example, heptanone-2, methyl salicylate, α-clove Benzene, Benzaldehyde, Benzyl Alcohol, Benzyl Acetate, Methoxybenzaldehyde, Yulanene, and Piperin.
Clove has an antibacterial effect. Ether extract of clove with 1% concentration, water immersion or decoction of Saboura's medium of clove with 8% concentration could inhibit the trichophyton schoenleinii, candida albicans and other pathogenic fungi. Clove oil and eugenol have a strong inhibition on the brucellosis, mycobacterium tuberculosis in the test tube. Clove oil and eugenol also have significant inhibitory effects on common pathogenic fungi. Clove oil and eugenol have antibacterial effect on the staphylococcus aureus, pneumonia, dysentery, large intestine, deformation and other bacteriat with the concentration 1: 2000~1: 8000.

Picture of lilac
Eugenin
Eugenin is naturally present in essential oils such as ylang ylang oil, Tuberose, jonquil oil and calamus oil. Eugenin is heavy with scent of flowers and clove. It has two isomers. The trans-isomer has a melting point of 33~ 34 ° C, and a boiling point of 140 ° C/1.6 Kpa, 118 ° C/670 Pa. The cis-isomer is a liquid having a boiling point of 115 ° C/670 Pa and 98 ° C/130 Pa. The product is a mixture of cis-isomer and trans-isomer, of with the majority is trans-isomers. The ratio of trans-isomer and cis-isomer is about 85:15, and the freezing point is about 12 ℃. According to the information provided by RIFM, the acute toxicity data of eugenol: Oral LD501.56 g/kg (rat), 1.41 g/kg (guinea pig). Cis-isomer has a LD50 of 0.365 g/kg and a trans-form of 0.54 g/kg. The highest doses of cis-isomer and trans-form which did not cause death are 0.10 g/kg and 0.20 g/kg respectively, and the lowest doses which cause death of all animals are 0.60 g/kg and 0.80 g/kg respectively.
Eugenin can be widely used in cosmetics and soaps and flavors. However, allergy is present if the concentration of Eugenin is too high, so IFRA prescribes a maximum concentration with 1% in the essence formula, no more than 0.2% in consumer products, and no more than 0.5% in consumer products that are not in contact with the skin.
Content analysis
Method 1: gas chromatography (GT-10-4) with non-polar column. The content is determined by area percentage.
Method 2: According to determination of phenol (OT-37). It was placed in the water bath for 30 minutes with heating, and cooled at room temperature.
Toxicity
ADI 0~2.5 (FAO/WHO, 1994)
LD50 1930~2680mg/kg (rat, oral)
GRAS (FDA $ 184.1257, 2000
Use limit
FEMA (mg/kg): soft drink 1.4; cold drink 3.1 0 candy 32; baked goods 33, pudding 0.60; chewing gum 500.
Application
Eugenol has a strong bactericidal and local anti-corrosive effect. It could be used for dental caries as a local analgesic drug. Eugenol is an intermediate of some other spices. Derivatives are eugenol, methyl eugenol, methyl isobutyl eugenol, acetylbutanoic eugenol, acetyl tauer eugenol, and benzyl isobornylphenol. When the eugenol is heated in potassium hydroxide, the double bond of propenyl is rearranged to form α-propenyl conjugated to the benzene ring to obtain isobutanol. Then acetylation and mild oxidation of isobutanol and α-propenyl cleavage is carried out. Finally we get vanillin, which is the main ingredient of an important artificial flavoring. Eugenol can also be used to prepare isonicid that is the specific drug for treatment of tuberculosis. It also could lower blood pressure.
Production
Eugenol can be isolated from natural essential oils, also be synthesized by chemical method in industry. However, chemical synthesis method produces isomers. Boiling point of two isomers is very close, resulting in difficult separation. So isolation method is the main method at present.
Isolation method from natural essential oil:
Take perennial sub-shrub clove basil as raw material, we get the mixture of essential oil and water through steam distillation. Add 20% of sodium hydroxide into the mixture, and then distill with steam to remove non-acidic substances. The resulting solution of sodium eugenol is added to 30% sulfuric acid at 50 ° C and stirred to pH = 2~3 (water layer). After standing, separate the lower layer of lilac oil, and then we get eugenol product through reduced pressure distillation.
Chemical synthesis
Allyl bromide, o-methoxyphenol, anhydrous acetone and anhydrous potassium carbonate are added to the kettle and heated to reflux for several hours. After cooling, dilute with water and then extract with ether. The extract is washed with 10% sodium hydroxide and dried over anhydrous potassium carbonate. Recover diethyl ether and acetone after distillation at atmospheric pressure, and then distill under reduced pressure and collect fraction at 110~113 ℃ (1600Pa), finally we get o-methoxyphenyl allyl ether. The mixture is boiled and refluxed for 1 hour and then cooled. The resulting grease is dissolved in ether and extracted with 10% sodium hydroxide solution. The extract is acidified with hydrochloric acid and extracted with ether. Dry the extract over anhydrous sodium sulfate and recover the ether through air distillation, and finally we get product. We could also get product through one step reaction between o-methoxyphenol and allyl chloride with copper as catalyst at 100 ℃.
Take essential oils containing large amounts of eugenol, such as clove oil, as raw materials, and add 30% sodium hydroxide solution, and then add inorganic acid or carbon dioxide to precipitate. In addition, addition reaction between clove oil and sodium acetate is also available.
Eugenol could be prepared by synthetic methods, but it is generally isolated from plants or aromatic oils in industry. We could take clove basil which originating from Seychelles, Comoros as raw material. In 1965, it was introduced into China from the former Soviet Union. It is cultivated in the south of the Yangtze River. The clove basil content is the highest in spike, followed by leaves, and stems are the last. The main ingredient in the oil is eugenol, accounting for 60-70%. There are linalool, parachute, ocimene and so on. We could prepare eugenol by synthetic method, in which o-methoxyphenol reacts with bromopropene. And then rearrangement is carried out with heating.
Category
Pesticide
Toxic grading
Moderate toxicity
Acute toxicity
Oral-LD50: 1930 mg/kg; Oral-Mouse LD50: 3000 mg/kg
Stimulate data
Skin-Rabbit 100 mg/24 h severe
Storage characteristics
Ventilated warehouse, low temperature, dry; separated from food materials
Extinguishing agent
Dry powder, foam, sand.
Description
Sensitization to eugenol mainly occurs in those in dental professions. Eugenol is contained in the "fragrance mix".
Chemical Properties
Eugenol has a strong aromatic odor of clove and a spicy, pungent taste. It darkens and thickens on exposure to air.
Chemical Properties
Eugenol is the main component
of several essential oils; clove leaf oil and cinnamon leaf oilmay contain>90%.Eugenol occurs in small amounts in many other essential oils. It is a colorless to
slightly yellow liquid with a spicy, clove odor.
Catalytic hydrogenation (e.g., in the presence of noble metal catalysts) yields
dihydroeugenol. Isoeugenol is obtained fromeugenol by shifting the double bond.
Esterification and etherification of the hydroxy group of eugenol yield valuable
fragrance and flavor materials (e.g., eugenol acetate and eugenol methyl ether).
Chemical Properties
colourless to faintly yellow liquid with a strong odour of cloves
Occurrence
Reported found as a constituent in several volatile oils: clove oil, laurel and cinnamon leaf oil. Smaller amounts of eugenol are also present in the oil of camphor, Java citronella, California laurel and acacia flowers; remarkable amounts of eugenol are found in Ocimum sanctum (70%) and Ocimum gratissimum (60%). Eugenol is also found in the oil from violet flowers (21%); in some plants, eugenol probably occurs as glucoside. Reported found in apricot, citrus oils, raspberry, strawberry, tomato, anise, cinnamon (leaf, bark and roots), clove bud and stem, nutmeg, mace, pepper, smoked fish, beer, whiskey, grape wines, cocoa, mango, tarragon, laurel, myrtle leaf, and pimento berry and leaf.
Uses
blood volume expander
Uses
Eugenol is a dental compound which shows cytotoxicity to human oral squamous cell carcinoma and oral cells. When glucosylated, this compound exhibits anti-inflammatory activity.
Uses
analgesic (topical), antiseptic, antifungal
Uses
Eugenol is a flavoring obtained from clove oil and also found in car- nation and cinnamon leaves. it is a stable, light yellow-green liquid of clove odor. it is slightly soluble in water and miscible in alcohol. it should be stored in glass or tin, avoiding iron containers. it is used in spice oils for application in condiments and meats at 100–200 ppm and in baked goods and candy at approximately 30 ppm.
Uses
eugenol is a botanical fraction. It is anti-bacterial, anti-inflammatory, and pain relieving. It can also be used as a local, topical anesthetic and antiseptic. In a cosmetic formulation, it can mask odor or provide fragrance. eugenol is a yellow, oily liquid and is generally associated with clove oil. However, it is also found in nutmeg, cinnamon, and bay leaf.
Preparation
Since sufficient eugenol can be isolated from cheap essential oils, synthesis is not industrially important. Eugenol is still preferentially isolated from clove leaf and cinnamon leaf oil (e.g., by extraction with sodium hydroxide solution). Nonphenolic materials are then removed by steam distillation. After the alkaline solution is acidified at low temperature, pure eugenol is obtained by distillation.
Definition
ChEBI: A phenylpropanoid formally derived from guaiacol with an allyl chain substituted para to the hydroxy group.
Aroma threshold values
Detection: 6 to 100 ppb
General Description
Clear colorless pale yellow or amber-colored liquid. Odor of cloves. Spicy pungent taste.
Air & Water Reactions
Darkens and thickens on exposure to air. Also darkens with age. Eugenol may decompose on exposure to light. Insoluble in water.
Reactivity Profile
Eugenol is incompatible with strong oxidizers. This includes ferric chloride and potassium permanganate. Eugenol reacts with strong alkalis. Eugenol is incompatible with iron and zinc.
Hazard
Questionable carcinogen.
Fire Hazard
Eugenol is combustible.
Contact allergens
Eugenol is a fragrance allergen obtained from many natural sources. Occupational sensitization to eugenol may occur in dental profession workers. Eugenol is contained in “fragrance mix” and has to be listed by name in cosmetics within the EU.
Clinical Use
4-Allyl-2-methoxyphenol is obtained primarily from cloveoil. It is a pale-yellow liquid with a strong aroma of clovesand a pungent taste. Eugenol is only slightly soluble in waterbut is miscible with alcohol and other organic solvents.Eugenol possesses both local anesthetic and antiseptic activityand can be directly applied on a piece of cotton to relievetoothaches. Eugenol is also used in mouthwashes because ofits antiseptic property and pleasant taste. The phenol coefficientof eugenol is 14.4.
Anticancer Research
This compound was tested on a model of skin tumor induced by DMBA croton oilin Swiss mice. The eugenol affects the cellular proliferation by increasing apoptosiscellular death. There is evidence for a downregulation of c-myc, H-ras, and Bcl-2expression and an upregulation of p53, Bax, and active caspase-3 (Grondona et al.2014).
Safety Profile
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Human mutation data reported. A human skin irritant. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALLYL COMPOUNDS.
Synthesis
The oil containing eugenol is treated with a 3% aqueous solution of NaOH; the nonacid components are extracted with ether; the alkaline solution is acidified to isolate the phenols and subsequently is fractionally distilled under reduced pressure; to avoid the formation of emulsions, a pretreatment of the oil with tartaric acid is preferred; eugenol is the starting material in one of the syntheses for the preparation of vanillin.
Metabolism
No absorption of eugenol occurred within 2hr of application to the intact shaved skin of mice (Meyer & Meyer, 1959). Following ip injec tion of [14C]eugenol into rats, radioactivity was dis tributed in various organs and the presence of 14CO2 in the expired air indicated the demethylation of eugenol (Weinberg, Rabinowitz, Zanger & Gennaro, 1972). Over 70% of an oral dose of eugenol was excreted in the urine of rabbits (Schr?der & Vollmer, 1932).
Purification Methods
Fractional distillation of eugenol gives a pale yellow liquid which darkens and thickens on exposure to air. It should be stored under N2 at -20o. [Waterman & Priedster Recl Trav Chim Pays-Bas 48 1272 1929, Beilstein 6 H 961, 6 IV 6337.]
Eugenol Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | aroma@qdtrustagri.com | China | 299 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8056 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5866 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 646 | 58 |
Related articles
- Eugenol: Overview and its Biological Activities
- Eugenol from clove oil exhibits antioxidant and antimicrobial properties, scavenging free radicals and disrupting bacterial me....
- May 15,2024
- Application and Pharmacology of Eugenol
- Eugenol is a phenylpropane compound existing in Syringa plants. It is a liquid with lilac flower flavor. Its chemical name is ....
- Jun 30,2022
- Introduction of eugenol
- Eugenol (C10H12O2) (CAS No:?97-53-0) is a volatile phenolic constituent of clove essential oil obtained from Eugenia caryophyl....
- Jan 14,2022
View Lastest Price from Eugenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-19 | Eugenol
97-53-0
|
US $19.00-19.00 / KG | 1KG | 99.% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-18 | Eugenol
97-53-0
|
US $0.00-0.00 / kg | 1kg | 99%GC | 10 tons | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-09-13 | Eugenol
97-53-0
|
US $10.00-7.00 / kg | 1kg | 99.99% | 10 tons | Shandong Deshang Chemical Co., Ltd. |
97-53-0(Eugenol)Related Search:
1of4