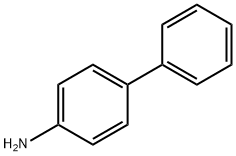
4-Aminobiphenyl
- CAS No.
- 92-67-1
- Chemical Name:
- 4-Aminobiphenyl
- Synonyms
- 4-Aminodiphenyl;4-PHENYLANILINE;[1,1'-BIPHENYL]-4-AMINE;4-adp;BIPHENYL-4-AMINE;biphenyl-4-ylamine;4-AMINOBIPHENYL, 100MG, NEAT;Of PCB;NSC 7660;Xenylamin
- CBNumber:
- CB7229899
- Molecular Formula:
- C12H11N
- Molecular Weight:
- 169.22
- MDL Number:
- MFCD00007879
- MOL File:
- 92-67-1.mol
| Melting point | 52-54 °C(lit.) |
|---|---|
| Boiling point | 191 °C15 mm Hg(lit.) |
| Density | 1.1154 (rough estimate) |
| vapor pressure | 6 x 10-5 mmHg at 20–30 °C (quoted, Mercer et al., 1990) |
| refractive index | 1.5785 (estimate) |
| Flash point | >110°C |
| storage temp. | -20°C Freezer |
| solubility | Soluble in Dichloromethane, DMSO and Methanol |
| pka | 4.35(at 18℃) |
| form | powder |
| color | Brown to Dark Yellow |
| Water Solubility | 842 mg/L at 20–30 °C (quoted, Mercer et al., 1990) |
| Merck | 13,1235 |
| BRN | 386533 |
| Henry's Law Constant | (x 1010 atm?m3/mol): 3.89 at 25 °C (calculated, Mercer et al., 1990) |
| Stability | Stable, but slowly reacts with oxygen in the air. Combustible. Incompatible with strong oxidizing agents, acids, acid anhydrides, acid chlorides. |
| InChIKey | DMVOXQPQNTYEKQ-UHFFFAOYSA-N |
| CAS DataBase Reference | 92-67-1(CAS DataBase Reference) |
| EWG's Food Scores | 6 |
| FDA UNII | 16054949HJ |
| Proposition 65 List | 4-Aminobiphenyl (4-aminodiphenyl) |
| IARC | 1 (Vol. 1, Sup 7, 99, 100F) 2012 |
| NIST Chemistry Reference | 4-Aminobiphenyl(92-67-1) |
| EPA Substance Registry System | 4-Aminobiphenyl (92-67-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H350 | |||||||||
| Precautionary statements | P201-P202-P264-P270-P301+P312-P308+P313 | |||||||||
| Hazard Codes | T,Xn | |||||||||
| Risk Statements | 45-22-36/37/38-20/21/22 | |||||||||
| Safety Statements | 53-45-36-26 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DU8925000 | |||||||||
| Autoignition Temperature | 842 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29214990 | |||||||||
| Toxicity | Acute oral LD50 for rats 200 mg/kg, mice 50 mg/kg (quoted, Verschueren, 1983). | |||||||||
| NFPA 704 |
|
4-Aminobiphenyl price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A2898 | 4-Aminobiphenyl | 92-67-1 | 1g | $40.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | A2898 | 4-Aminobiphenyl | 92-67-1 | 5g | $96.9 | 2024-03-01 | Buy |
| Alfa Aesar | H55424 | 4-Aminobiphenyl, 98% | 92-67-1 | 1g | $19.9 | 2023-06-20 | Buy |
| Alfa Aesar | H55424 | 4-Aminobiphenyl, 98% | 92-67-1 | 5g | $62.9 | 2023-06-20 | Buy |
| Sigma-Aldrich | 31598 | 4-Aminobiphenyl analytical standard | 92-67-1 | 250mg | $72.7 | 2024-03-01 | Buy |
4-Aminobiphenyl Chemical Properties,Uses,Production
Description
4-Aminobiphenyl is an aromatic amine (arylamine) that exists at room temperature as a colorless crystalline solid with a floral odor. It is slightly soluble in cold water, but readily soluble in hot water. It is soluble in ethanol, ether, acetone, chloroform, and lipids. It oxidizes in air and emits toxic fumes when heated to decomposition (Akron 2009).
Physical properties
Colorless to yellow-brown crystalline solid with a floral-like odor. Becomes purple on exposure to air.
Uses
Previously used as a rubber antioxidant; no longer produced on a commercial scale
Uses
In the detection of sulfates. Formerly as rubber antioxidant. As carcinogen in cancer research, induces chromosomal instability in human cancer cells.
Uses
In the United States, 4-aminobiphenyl now is used only in laboratory research. It formerly was used commercially as a rubber antioxidant, as a dye intermediate, and in the detection of sulfates (HSDB 2009).
Definition
ChEBI: 4-Aminobiphenyl is an aminobiphenyl that is biphenyl substituted by an amino group at position 4. It has a role as a carcinogenic agent. It derives from a hydride of a biphenyl.
Synthesis Reference(s)
Journal of the American Chemical Society, 97, p. 7184, 1975 DOI: 10.1021/ja00857a049
Tetrahedron Letters, 39, p. 1313, 1998 DOI: 10.1016/S0040-4039(97)10877-2
General Description
Colorless to yellowish-brown crystals or light brown solid.
Air & Water Reactions
Is oxidized by air (darkens on oxidation). Insoluble in water.
Reactivity Profile
4-AMINOBIPHENYL is a weak base. Incompatible with acids and acid anhydrides. Forms salts with hydrochloric acid and sulfuric acid. Can be diazotized, acetylated and alkylated. . May react with strong oxidizing agents.
Hazard
Toxic by ingestion, inhalation, skin absorp- tion. Confirmed carcinogen. Bladder and liver can- cer.
Health Hazard
4-Aminodiphenyl exposure is associated with a high incidence of bladder cancer in humans.
Fire Hazard
4-AMINOBIPHENYL is probably combustible.
Safety Profile
Confirmed human carcinogen with experimental carcinogenic and tumorigenic data. Poison by ingestion and intraperitoneal routes. Human mutation data reported. An irritant. Effects resemble those of benzidine. See also BENZIDINE. Slight to moderate fire hazard when exposed to heat, flames (sparks), or powerful oxidzers. To fight fire, use water spray, mist, dry chemical. When heated to decomposition it emits toxic fumes of NOx,. See also AROMATIC AMINES.
Synthesis
4-aminobiphenyl is prepared by reduction of 4-nitrobiphenyl, which, together with the 2-nitro derivatives, is obtained by nitration of biphenyl.
Potential Exposure
4-Aminobiphenyl is no longer manufactured commercially and is only used for research purposes. 4-Aminobiphenyl was formerly used as a rubber antioxidant and as a dye intermediate. Is a contaminant in 2-aminobiphenyl.
Carcinogenicity
4-Aminobiphenyl is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
Cancer of the urinary bladder was first reported to be associated with occupational exposure to 4-aminobiphenyl in a descriptive epidemiological study (published in the mid 1950s), in which 11% (19 of 171) of workers in a plant manufacturing 4-aminobiphenyl developed urinary-bladder cancer. These workers had been exposed to 4-aminobiphenyl for 1.5 to 19 years between 1935 and 1955. Publication of this study led to an effort to discontinue production and useof 4-aminobiphenyl. Starting in 1955, 541 workers who had been exposed to 4-aminobiphenyl were followed for an additional 14 years; 43 men (7.9%) developed histologically confirmed urinary-bladder cancer. In a survey of workers at a plant producing a variety of chemicals, the risk of mortality from urinary-bladder cancer was elevated tenfold, and all of the men who died of urinary-bladder cancer had worked at the plant during the period when 4-aminobiphenyl was used (1941 through 1952). The International Agency for Research onCancer concluded that there was sufficient evidence of the carcinogenicity of 4-aminobiphenyl in humans (IARC 1972, 1987).
Since 4-aminobiphenyl was listed in the First Annual Report on Carcinogens, most research on its carcinogenicity has focused on exposure from cigarette smoking. Epidemiological studies have reported the incidence of urinary-bladder cancer to be 2 to 10 times as high among cigarette smokers as among nonsmokers. Higher levels of 4-aminobiphenyl adducts (4-aminobiphenyl metabolites bound to DNA or protein) were detected in bladder tumors (DNA adducts) and red blood cells (hemoglobin adducts) from smokers thanfrom nonsmokers (Feng et al. 2002). In a case-control study, levels of 4-aminobiphenyl–hemoglobin adducts were higher in smokers with urinary-bladder cancer than in a control group of similarly exposed smokers (Del Santo et al. 1991). A Taiwanese study reported that 4-aminobiphenyl–hemoglobin adducts were associated with increased risk of liver cancer (Wang et al. 1998).
Environmental Fate
4-Aminobiphenyl is one of a number of chemicals that cause methemoglobinemia, or conversion of hemoglobin to methemoglobin, which reduces the ability of the blood to carry oxygen to the tissues. In addition, the active metabolite is believed to produce cancer through its reaction with cellular DNA. In animal studies, the observed incidence of 4-aminobiphenyl adducts with bladder epithelium DNA correlated well with the observed bladder tumor incidence.
Metabolic pathway
Ring oxidation of 4-aminobiphenyl occurred only to a minor extent in microsomes. In contrast, N-oxidation of 4,4'-methylene-bis-(2-chloroaniline) is preferentially catalyzed by the phenobarbital-induced enzymes P- 450PB-B and P-450PB-D to cause ring oxidation and methylene carbon oxidation. 4,4'-Methylene-bis-(2- chloroaniline) ring oxidation and methylene carbon oxidation show varied cytochrome P-450 selectivity and accounted for 14-79% of total oxidation products.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3143 Dyes, solid, toxic, n.o.s. or Dye intermediates, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from water or EtOH. [Beilstein 12 IV 3241.] CARCINOGENIC.
Toxicity evaluation
4-Aminobiphenyl may have been released into the environment during its production and use as a rubber antioxidant and dye intermediate; however, sources suggest that it was no longer in significant production by the early 1970s. 4-Aminobiphenyl is easily oxidizable and probably undergoes photolysis but there is little actual data on these processes. If released on land it is expected to adsorb moderately to soil, probably binding to humic materials, and undergo redox reactions. If released to surface water, it is expected to adsorb to sediment, and probably undergo photolysis and oxidation. It may be degraded by oxidation by alkoxy radicals, which are photochemically produced in eutrophic waters, with an estimated half-life of 14 days. 4-Aminobiphenyl is biodegradable and biodegradation may well occur in both soil and water but there are no rates available for soil or surface waters. It has a low potential for bioconcentration. In the atmosphere, degradation should occur due to direct photolysis, oxidation by ambient oxygen, and photochemically produced hydroxyl radicals (estimated half-life 6–7 h in the vapor phase).
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides and acid anhydrides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal devices.
4-Aminobiphenyl Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Baynoe Chem (Suzhou) Co., Ltd. | +86-512 65869404 +86-18934593683 | sales06@baynoe.com | China | 292 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1100 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
Related articles
- Toxicity of 4-Aminobiphenyl
- 4-Aminobiphenyl was formerly used as a rubber antioxidant and in the manufacture of azo dyes. Due to its carcinogenicity 4-ami....
- Nov 4,2021
View Lastest Price from 4-Aminobiphenyl manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-02-23 | 4-Aminodiphenyl
92-67-1
|
US $345.00 / kg | 1kg | 0.995 | 25kg | Shanghai UCHEM Inc. | |
 |
2023-11-08 | 4-Aminobiphenyl
92-67-1
|
US $35.00-25.00 / kg | 1kg | 99% | 20 Ton | Hebei Xinsheng New Material Technology Co., LTD. | |
 |
2023-09-14 | 4-Aminobiphenyl
92-67-1
|
US $100.00 / bag | 1bag | 99 | 5000 | Hebei Fengqiang Trading Co., LTD |
-

- 4-Aminodiphenyl
92-67-1
- US $345.00 / kg
- 0.995
- Shanghai UCHEM Inc.
-

- 4-Aminobiphenyl
92-67-1
- US $35.00-25.00 / kg
- 99%
- Hebei Xinsheng New Material Technology Co., LTD.
-

- 4-Aminobiphenyl
92-67-1
- US $100.00 / bag
- 99
- Hebei Fengqiang Trading Co., LTD
92-67-1(4-Aminobiphenyl)Related Search:
1of4