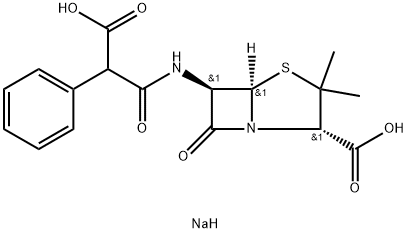
Carbenicillin disodium
- CAS No.
- 4800-94-6
- Chemical Name:
- Carbenicillin disodium
- Synonyms
- geopen;pyopen;hyoper;piopen;pyopene;gripenin;Carbapen;brl-2064;carbecin;ANABACTYL
- CBNumber:
- CB7308325
- Molecular Formula:
- C17H19N2NaO6S
- Molecular Weight:
- 402.4
- MDL Number:
- MFCD00077683
- MOL File:
- 4800-94-6.mol
- MSDS File:
- SDS
| Melting point | >190°C (dec.) |
|---|---|
| alpha | +175~+190°(D/20℃)(c=4,H2O)(calculated on the dehydrous basis) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white to off-white |
| PH | 5.5~7.5 (10g/l, 25℃) |
| Water Solubility | Soluble in water. |
| Merck | 13,1801 |
| BRN | 5722128 |
| FDA UNII | 9TS4B3H261 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P272-P280-P284-P302+P352-P333+P313 |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-36 |
| RIDADR | UN 1170 3/PG 3 |
| WGK Germany | 2 |
| RTECS | ON9105000 |
| F | 3-8-10 |
| HS Code | 29411000 |
| Toxicity | LD50 i.p. in rats: >2000 mg/kg (Goldenthal) |
Carbenicillin disodium price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C1389 | Carbenicillin disodium salt 89.0-100.5% anhydrous basis | 4800-94-6 | 1g | $130 | 2024-03-01 | Buy |
| Sigma-Aldrich | C1389 | Carbenicillin disodium salt 89.0-100.5% anhydrous basis | 4800-94-6 | 5g | $449 | 2024-03-01 | Buy |
| Alfa Aesar | J61949 | Carbenicillin disodium salt | 4800-94-6 | 1g | $94.1 | 2024-03-01 | Buy |
| Alfa Aesar | J61949 | Carbenicillin disodium salt | 4800-94-6 | 5g | $277 | 2024-03-01 | Buy |
| Cayman Chemical | 20871 | Carbenicillin (sodium salt) | 4800-94-6 | 5g | $81 | 2024-03-01 | Buy |
Carbenicillin disodium Chemical Properties,Uses,Production
Description
Carbenicillin is a broad-spectrum carboxypenicillin antibiotic. It is active against Gram-negative and certain Gram-positive bacteria, including S. pyogenes, S. epidermidis, P. mirabilis, P. vulgaris, E. coli, and P. aeruginosa (MICs = 0.19, 1.56, 1.56, 3.12, 3.12, and 50 μg/ml, respectively). It is also active against penicillinase-producing and non-producing strains of S. aureus (MICs = 1.56 and 12.5 μg/ml, respectively). Carbenicillin is protective against systemic S. pyogenes, P. vulgaris, E. coli, and S. aureus infection in a mouse model of systemic lethal infection with 50% protective dose (PD50) values of 7.8, 224, 19.3, and 34 mg/kg, respectively. It also decreases viable colony counts in the kidney in a rat model of P. vulgaris or E. coli urinary tract infection when administered at a dose of 100 mg/kg. Formulations containing carbenicillin have previously been used in the treatment of upper and lower urinary tract infections and prostatitis.
Chemical Properties
white Powder
Originator
Pyopen,Beecham,Switz.,1968
Uses
Carbenicillin disodium salt is a water-soluble antibiotic which is effective against gram-negative bacteria.Carbenicillin disodium salt is widely utilized as an antibiotic which is used to control bacterial cell-wall synthesis by inactivating transpeptidases on the inner surface of the bacterial cell membrane. It has been involved in the study of the role of penicillin-sensitive transpeptidases in cell wall biosynthesis.
Uses
Semi-synthetic antibiotic related to Penicillin (P223500). Antibacterial.
Definition
ChEBI: Carbenicillin disodium is an organic sodium salt. It contains a carbenicillin(2-).
Manufacturing Process
The required monobenzyl phenylmalonate, MP 68°C, was prepared by treating
a mixture of phenylmalonic acid (18 g) and benzyl alcohol (13 g) in carbon
tetrachloride (80 ml) with dry hydrogen chloride.
Monobenzyl phenylmalonate (13.3 g) in dry benzene (100 ml) was refluxed with thionyl chloride (6.45 g) for 90 minutes, then concentrated in vacuo. The
residual oil was dissolved in dry acetone (50 ml) and added to a stirred, ice-cooled solution of 6-aminopenicillanic acid (9.7 g) in N sodium bicarbonate
solution (135 ml), water (150 ml), and acetone (300 ml). The mixture was
stirred for 30 minutes at 0°C and then for 90 minutes at room temperature,
then concentrated under reduced pressure to remove acetone. The aqueous
solution was brought to pH 2 with dilute hydrochloric acid and extracted with
ether (3 x 100 ml). The ether solution was washed with water and then itself
extracted with sufficient N sodium bicarbonate solution to give an aqueous
phase of pH 7.5. The aqueous layer was separated and evaporated at low
temperature and pressure to leave the impure sodium salt of alpha-
(benzyloxycarbonyl) benzylpenicillin.
This crude product (15.8 g) in water (360 ml) was added to a
prehydrogenated suspension of 10% palladium on charcoal (4 g) in water
(400 ml), and hydrogenation was continued for 30 minutes. The catalyst was
removed and the filtrate was adjusted to pH 7.5 with sodium bicarbonate,
then evaporated at low temperature and pressure. The residue was purified by
chromatography on a column of cellulose powder, eluting first with
butanol/ethanol/water mixture and then with acetone/isopropanol/water. The
main fraction was evaporated at low temperature and pressure to give a 32%
yield of the sodium salt of alpha-carboxybenzylpenicillin as a white powder.
The product was estimated by monometric assay with penicillinase to be 58%
pure.
brand name
Geopen (Roerig); Pyopen (GlaxoSmithKline).
Therapeutic Function
Antibacterial
General Description
Carbenicillin was synthesized by Brain et al. of Beecham Research Laboratories in 1965. It was the first synthetic penicillin to show activity against Pseudomonas aeruginosa. Although its activity against the microorganism is not strong (MIC = 25 – 100 μg/mL), it is widely used against P. aeruginosa infections because of its low toxicity and the lack of other antibiotics suitable for use against this microorganism. Carbenicillin is mainly used clinically to treat urinary tract and respiratory tract infections and sepsis caused by Proteus, Escherichia coli, Klebsiella, and Pseudomonas aeruginosa.
Clinical Use
Carbenicillin disodium, disodium α-carboxybenzylpenicillin(Geopen, Pyopen), is a semisynthetic penicillin releasedin the United States in 1970, which was introduced inEngland and first reported by Ancred et al. in 1967.Examination of its structure shows that it differs from ampicillinin having an ionizable carboxyl group rather than anamino group substituted on the α-carbon atom of the benzylside chain. Carbenicillin has a broad range of antimicrobialactivity, broader than any other known penicillin, a propertyattributed to the unique carboxyl group. It has been proposedthat the carboxyl group improves penetration of themolecule through cell wall barriers of Gram-negativebacilli, compared with other penicillins.
Carbenicillin is not stable in acids and is inactivated bypenicillinase. It is a malonic acid derivative and, as such, decarboxylatesreadily to penicillin G, which is acid labile.Solutions of the disodium salt should be freshly prepared but,when refrigerated, may be kept for 2 weeks. It must be administeredby injection and is usually given intravenously.
Carbenicillin has been effective in the treatment of systemicand urinary tract infections caused by P. aeruginosa,indole-producing Proteus spp., and Providencia spp., all ofwhich are resistant to ampicillin. The low toxicity of carbenicillin,with the exception of allergic sensitivity, permits theuse of large dosages in serious infections. Most cliniciansprefer to use a combination of carbenicillin and gentamicinfor serious pseudomonal and mixed coliform infections. Thetwo antibiotics are chemically incompatible, however, andshould never be combined in an intravenous solution.
Carbenicillin disodium Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Suzhou Actchem Co., Ltd. | +8618762124502 | actchem@qq.com | China | 27 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3783 | 58 |
View Lastest Price from Carbenicillin disodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-30 | Carbenicillin disodium
4800-94-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-03-06 | Carbenicillin disodium
4800-94-6
|
US $10.50 / KG | 1KG | 99% | 1 ton | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2022-05-14 | Carbenicillin disodium
4800-94-6
|
US $1.10 / g | 1g | 99.9% Min | 100 Tons | Dideu Industries Group Limited |
-

- Carbenicillin disodium
4800-94-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Carbenicillin disodium
4800-94-6
- US $10.50 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Carbenicillin disodium
4800-94-6
- US $1.10 / g
- 99.9% Min
- Dideu Industries Group Limited
4800-94-6(Carbenicillin disodium)Related Search:
1of4