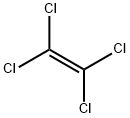
Tetrachloroethylene
- CAS No.
- 127-18-4
- Chemical Name:
- Tetrachloroethylene
- Synonyms
- PERCHLOROETHYLENE;PCE;PERCHLORETHYLENE;Nema;TETRACHLOROETHENE;PERC;C2Cl4;Tetrachlorethylene;Perk;Tetrachlorethen
- CBNumber:
- CB7325193
- Molecular Formula:
- C2Cl4
Lewis structure
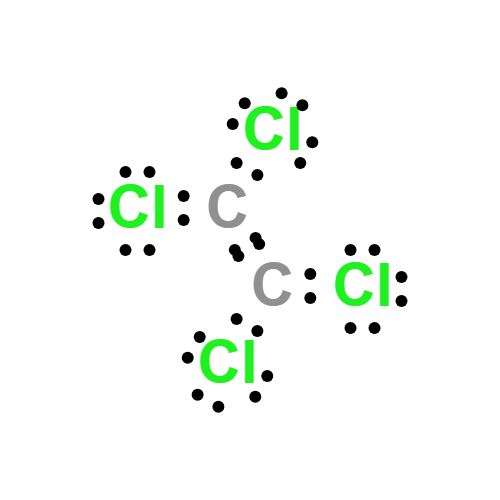
- Molecular Weight:
- 165.83
- MDL Number:
- MFCD00000834
- MOL File:
- 127-18-4.mol
- MSDS File:
- SDS
| Melting point | -22 °C (lit.) |
|---|---|
| Boiling point | 121 °C (lit.) |
| Density | 1.623 g/mL at 25 °C (lit.) |
| vapor density | 5.83 (vs air) |
| vapor pressure | 13 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 120-121°C |
| storage temp. | Store at +2°C to +25°C. |
| solubility | water: soluble0.15g/L at 25°C |
| form | Liquid |
| color | APHA: ≤10 |
| Odor | chloroform-like odor |
| Odor Threshold | 0.77ppm |
| Evaporation Rate | 0.33 |
| Water Solubility | Miscible with alcohol, ether, chloroform, benzene and hexane. Slightly miscible with water. |
| FreezingPoint | -22.0℃ |
| λmax |
λ: 290 nm Amax: 1.00 λ: 295 nm Amax: 0.30 λ: 300 nm Amax: ≤0.20 λ: 305 nm Amax: 0.10 λ: 350 nm Amax: 0.05 λ: 400 nm Amax: 0.03 |
| Merck | 14,9190 |
| BRN | 1361721 |
| Henry's Law Constant | 4.97 at 1.8 °C, 15.5 at 21.6 °C, 34.2 at 40.0 °C, 47.0 at 50 °C, 68.9 at 60 °C, 117.0 at 70 °C (EPICS-GC, Shimotori and Arnold, 2003) |
| Exposure limits | TLV-TWA 50 ppm (~325 mg/m3) (ACGIH), 100 ppm (MSHA and OSHA); TLV-STEL 200 ppm (ACGIH); carcinogenicity: Animal Limited Evidence. |
| Dielectric constant | 2.5(21℃) |
| Stability | Stable. Incompatible with strong oxidizing agents, alkali metals, aluminium, strong bases. |
| EPA Primary Drinking Water Standard | MCL:0.005,MCLG:zero |
| LogP | 2.53 at 20℃ |
| Indirect Additives used in Food Contact Substances | TETRACHLOROETHYLENE |
| FDA 21 CFR | 175.105; 178.3010 |
| CAS DataBase Reference | 127-18-4(CAS DataBase Reference) |
| EWG's Food Scores | 7-10 |
| FDA UNII | TJ904HH8SN |
| Proposition 65 List | Tetrachloroethylene (Perchloroethylene) |
| NIST Chemistry Reference | Tetrachloroethylene(127-18-4) |
| IARC | 2A (Vol. Sup 7, 63, 106) 2014 |
| EPA Substance Registry System | Tetrachloroethylene (127-18-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H317-H319-H336-H351-H411 | |||||||||
| Precautionary statements | P201-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,N,T,F | |||||||||
| Risk Statements | 40-51/53-23/25-11-39/23/24/25-23/24/25 | |||||||||
| Safety Statements | 23-36/37-61-45-24-16-7 | |||||||||
| RIDADR | UN 1897 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KX3850000 | |||||||||
| Autoignition Temperature | 260℃ | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29032300 | |||||||||
| Toxicity | LD50 orally in mice: 8.85 g/kg (Dybing); LC for mice in air: 5925 ppm (Lazarew) | |||||||||
| IDLA | 150 ppm | |||||||||
| NFPA 704 |
|
Tetrachloroethylene price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 48609 | Tetrachloroethene solution certifiedreferencematerial,200?μg/mLinmethanol | 127-18-4 | 1mL | $24.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40083 | Tetrachloroethene solution certifiedreferencematerial,5000?μg/mLinmethanol | 127-18-4 | 1mL | $46.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.00964 | Tetrachloroethylene EMPLURA? | 127-18-4 | 1L | $134 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.00965 | Tetrachloroethylene for spectroscopy Uvasol? | 127-18-4 | 500mL | $195 | 2024-03-01 | Buy |
| Sigma-Aldrich | 15889 | Density Standard 1623 kg/m3 H&DFitzgeraldLtd.Quality | 127-18-4 | 10ml | $280 | 2024-03-01 | Buy |
Tetrachloroethylene Chemical Properties,Uses,Production
Description
Tetrachloroethylene (chemical formula Cl2C=CCl2) is a chlorinate hydrocarbon used as an industrial solvent and cooling liquid in electrical transformers. It is a colorless, volatile, nonflammable liquid with an ether-like odor. The major part of tetrachloroethylene is produced by high temperature chlorinolysis of light hydrocarbons.

Tetrachloroethylene is an excellent solvent for organic materials. It is volatile, highly stable, and nonflammable, and thus being widely used in dry cleaning. It can also be used to degrease metal parts in the automotive and other metalworking industries upon being mixed with other chlorocarbons. It can also be used in neutrino detectors. However, it should be noted that it is a potential carcinogen.
Chemical Properties
Tetrachloroethylene is a clear, colorless, volatile, nonflammable liquid with an ethereal odor. Insoluble in water. Vapors heavier than air. Density approximately 13.5 lb/gal. Used as dry cleaning solvent, a degreasing solvent, a drying agent for metals, and in the manufacture of other chemicals.
Uses
Tetrachloroethylene (PCE) is also known as perchloroethylene, tetrachloroethene, and 1,1,2,2- tetrachloroethene and is also commonly abbreviated to PER or PERC. Tetrachloroethylene is a volatile, chlorinated organic hydrocarbon that is widely used as a solvent in the dry-cleaning and textile-processing industries and as an agent for degreasing metal parts. It is an environmental contaminant that has been detected in the air, groundwater, surface waters, and soil (NRC, 2010).
References
https://en.wikipedia.org/wiki/Tetrachloroethylene
https://pubchem.ncbi.nlm.nih.gov/compound/tetrachloroethylene#section=Top
Description
Perchloroethylene (Tetrachloroethylene) is a colourless liquid with a slightly ethereal odour. It is marginally soluble in water and soluble in most organic solvents.Perchloroethylene has a limited number of uses and applications. It is used as intermediate, as dry cleaning agent in the industrial and professional sector, as surface cleaning agent in industrial settings, as heat transfer medium in industrial settings, and in film cleaning and copying by professionals. It is also used as a chemical intermediate in the production of fluorinated compounds and in industrial surface cleaning metal degreasing. Occupational exposure to perchloroethylene is possible in the manufacturing facilities or the industrial facilities where it is used as an intermediate.
Chemical Properties
Tetrachloroethylene is a clear, colorless, nonflammable liquid with a characteristic odor. The odor is noticeable @ 47 ppm, though after a short period it may become inconspicuous, thereby becoming an unreliable warning signal. The Odor Threshold is variously given as 5 ppm to 6.17 (3M).
Physical properties
Clear, colorless, nonflammable liquid with a chloroform or sweet, ethereal odor. Odor threshold concentration is 4.68 ppmv (Leonardos et al., 1969). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.24 and 2.8 mg/L, respectively (Alexander et al., 1982).
Uses
Tetrachloroethylene is used as a solvent, indrycleaning, and in metal degreasing.Tetrachloroethylene is a common industrial solvent that is often found as a contaminant in groundwater. Tetrachloroethylene is also a suspected carcinogen to humans and is difficult to degrade biologically as it has no natural source. This compound is a contaminant of emerging concern (CECs).
Production Methods
Tetrachloroethylene (PCE) was first prepared in 1821 and commercial production in the United States began in 1925. Several commercial grades are available that differ in the amount and type of added stabilizers (e.g., amines, phenols, and epoxides).
The industrial processes for production of tetrachloroethylene include threetechnical routes:
(1) chlorination of trichloroethylene and followed by dehydrochlorination.
(2) oxychlorination of ethylene.
(3) chlorination andpyrolysis of light hydrocarbon.
In China, the chlorination of trichloroethylene and dehydrochlorination process is mainly used for production of tetrachloroethylene, while the other two processes are widely used in other countries.
Definition
ChEBI: A chlorocarbon that is tetrachloro substituted ethene.
Synthesis Reference(s)
Journal of the American Chemical Society, 90, p. 5307, 1968 DOI: 10.1021/ja01021a065
General Description
Tetrachloroethylene (perchloroethylene, PCE), is a chlorinated ethylene compound commonly used as a dry cleaning and degreasing solvent. It shows IR transparency as it has no C–H bonds making it an ideal solvent for IR spectroscopy. PCE is a man-made pollutant which is difficult to degrade. It is a ground water contaminant which has adverse effect on human health due to its potential toxicity and carcinogenicity. Some of the methods proposed for its degradation are Fenton oxidation treatment, reductive dehalogenation under methanogenic condition, and reduction using zero valent metal ions. One of the methods reported for its synthesis is from ethylene dichloride and chlorine.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Tetrachloroethylene decomposes upon heating and exposure to UV light to give phosgene and HCl. Reacts violently with finely dispersed light metals (aluminum) and zinc. [Handling Chemicals Safely 1980 p. 887]. Mixtures with finely divided barium or lithium metal can detonate [ASESB Pot. Incid. 39. 1968; Chem. Eng. News 46(9):38. 1968]. Decomposes very slowly in water to form trichloroacetic acid and hydrochloric acid
Health Hazard
Exposure to tetrachloroethylene can produceheadache, dizziness, drowsiness, incoordina tion, irritation of eyes, nose, and throat, and flushing of neck and face. Exposure to highconcentrations can produce narcotic effects.The primary target organs are the centralnervous system, mucous membranes, eyes,and skin. The kidneys, liver, and lungs areaffected to a lesser extent. Symptoms ofdepression of the central nervous system aremanifested in humans from repeated expo sure to 200 ppm for 7 hours/day. Chronicexposure to concentrations ranging from 200to 1600 ppm caused drowsiness, depression,and enlargement of the kidneys and livers inrats and guinea pigs. A 4-hour exposure to4000 ppm of vapor in air was lethal to rats.
Ingestion of tetrachloroethylene may pro duce toxic effects ranging from nausea andvomiting to somnolence, tremor, and ataxia.The oral toxicity, is low, however, with LD50ranging between 3000 and 9000 mg/kg inanimals. Skin contact with the liquid maycause defatting and dermatitis of skin.
Evidence of carcinogenicity of this com pound has been noted in test animals sub jected to inhalation or oral administration. Itcaused tumors in the blood, liver, and kidneyin rats and mice. Carcinogenicity in humansis not reported.
Fire Hazard
Special Hazards of Combustion Products: Toxic, irritating gases may be generated in fires.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Animal carcinogen that produces increased incidence of renal adenomas, adenocarcinomas, mononuclear cell leukemia, and hepatocellular tumors.
Safety Profile
Tetrachloroethylene is a nonflammable colorless liquid with a sharp sweet odor. Tetrachloroethylene is widely used for dry-cleaning fabrics and metal degreasing operations. Effects resulting from acute (short term) high-level inhalation exposure of humans to tetrachloroethylene include irritation of the upper respiratory tract and eyes, kidney dysfunction, and neurological effects such as reversible mood and behavioral changes, impairment of coordination, dizziness, headache, sleepiness, and unconsciousness. The primary effects from chronic (long term) inhalation exposure are neurological, including impaired cognitive and motor neurobehavioral performance. Tetrachloroethylene exposure may also cause adverse effects in the kidney, liver, immune system and hematologic system, and on development and reproduction. Studies of people exposed in the workplace have found associations with several types of cancer including bladder cancer, non-Hodgkin lymphoma, multiple myeloma. U.S. EPA has classified tetrachloroethylene as likely to be carcinogenic to humans.
Potential Exposure
Tetrachloroethylene is used in the textile industry and as a chemical intermediate or a heatexchange fluid; a widely used solvent with particular use as a dry cleaning agent; a degreaser; a fumigant, and medically as an anthelmintic.
Carcinogenicity
Tetrachloroethylene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN1897 Tetrachloroethylene, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
It decomposes under similar conditions to CHCl3, to give phosgene and trichloroacetic acid. Inhibitors of this reaction include EtOH, diethyl ether and thymol (effective at 2-5ppm). Tetrachloroethylene should be distilled under a vacuum (to avoid phosgene formation) and stored in the dark out of contact with air. It can be purified by washing with 2M HCl until the aqueous phase no longer becomes coloured, then with water, drying with Na2CO3, Na2SO4, CaCl2 or P2O5, and fractionally distilling just before use. 1,1,2-Trichloroethane and 1,1,1,2-tetrachloroethane can be removed by counter-current extraction with EtOH/water. [Beilstein 1 IV 715.]
Incompatibilities
Violent reaction with strong oxidizers; powdered, chemically active metals, such as aluminum, lithium, beryllium, and barium; caustic soda; sodium hydroxide; potash. Tetrachloroethylene is quite stable. However, it reacts violently with concentrated nitric acid to give carbon dioxide as a primary product. Slowly decomposes on contact with moisture producing trichloroacetic acid and hydrochloric acid. Decomposes in UV light and in temperatures above 150℃, forming hydrochloric acid and phosgene.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Alternatively, PCE may be recovered from waste gases and reused.
Tetrachloroethylene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15748 | 58 |
| BLiT (Hefei)Chemical Co.,Ltd | +86-19521662663 +86-19521662663 | lucius@blitchem.com | China | 291 | 58 |
| Qingdao Minzhi Yijie new material Co., LTD | +86-13589435123 +86-13589435123 | qdmzyj@126.com | China | 240 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 | 1012@dideu.com | China | 3758 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7038 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
Related articles
- Tetrachloroethylene: Uses and Effects on humans
- Tetrachloroethylene is mainly used as a dry-cleaning agent. It is an intermediate for production of trichloroacetic acid and f....
- Mar 20,2024
- Biological actions and interactions of tetrachloroethylene
- Tetrachloroethylene is a colorless, volatile, nonflammable, liquid, chlorinated hydrocarbon with an ether-like odor that may e....
- May 7,2022
- What is Perchloroethylene?
- Perchloroethylene (C2Cl4, CAS No: 127-18-4), also known as perc, is a colorless, nonflammable liquid solvent with a sweet, eth....
- Sep 27,2021
View Lastest Price from Tetrachloroethylene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-16 | Tetrachloroethylene
127-18-4
|
US $100.00-90.00 / kg | 1kg | 99% | 20Tons | Hebei Dangtong Import and export Co LTD | |
 |
2023-12-23 | Tetrachloroethylene
127-18-4
|
US $50.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-09-04 | Tetracchloroethylene
127-18-4
|
US $1.25-180.60 / Kg | 1Kg | 99%min | 300000Tons | Shaanxi Dideu Medichem Co. Ltd |
-

- Tetrachloroethylene
127-18-4
- US $100.00-90.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Tetrachloroethylene
127-18-4
- US $50.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Tetracchloroethylene
127-18-4
- US $1.25-180.60 / Kg
- 99%min
- Shaanxi Dideu Medichem Co. Ltd
127-18-4(Tetrachloroethylene)Related Search:
1of4