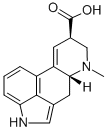
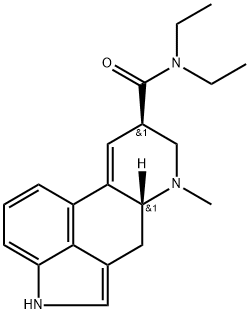
Lysergic acid diethylamide
- CAS No.
- 50-37-3
- Chemical Name:
- Lysergic acid diethylamide
- Synonyms
- LSA;LSD;Cubes;D-LSD;Ergine;LSD 25;lsd-25;C07542;Delysid;DNAS1L3
- CBNumber:
- CB7418655
- Molecular Formula:
- C20H25N3O
- Molecular Weight:
- 323.44
- MDL Number:
- MFCD00135795
- MOL File:
- 50-37-3.mol
| Melting point | 91-93?C |
|---|---|
| alpha | D20 +17° (c = 0.5 in pyridine) |
| Boiling point | 461.9°C (rough estimate) |
| Density | 1.1021 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| Flash point | 2℃ |
| storage temp. | −20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | pKa 7.5 (Uncertain) |
| color | Pale Brown to Brown |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| EWG's Food Scores | 1 |
| FDA UNII | 8NA5SWF92O |
| EPA Substance Registry System | Lysergide (50-37-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H312+H332-H319-H302+H312-H331 | |||||||||
| Precautionary statements | P210-P261-P302+P352+P312-P304+P340+P312-P337+P313-P403+P235-P403+P233 | |||||||||
| Hazard Codes | T+,T,F,Xn | |||||||||
| Risk Statements | 26/27/28-40-36/38-23/25-36-20/21/22-11 | |||||||||
| Safety Statements | 22-28-36-45-33-24-16-7-36/37-26 | |||||||||
| RIDADR | UN 2811 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KE4100000 | |||||||||
| Toxicity | LD50 in mice, rats, rabbits (mg/kg): 46, 16.5, 0.3 i.v. | |||||||||
| DEA Controlled Substances | CSCN: 7310 CAS SCH: III NARC: N |
|||||||||
| DEA Controlled Substances | CSCN: 7310 CAS SCH: III NARC: N |
|||||||||
| NFPA 704 |
|
Lysergic acid diethylamide Chemical Properties,Uses,Production
Description
Lysergic acid diethylamide (LSD) is synthetically derived from the fungus, Claviceps purpuria. Several isomers of this compound exist, although only the D-isomer is considered active. LSD was first synthesized by Albert Hofmann in 1938 in a search for an analeptic drug. After finding its psychogenic activity, it was marketed by Sandoz laboratories under the name Delysid?. It was also reportedly used by the Central Intelligence Agency as a tool for interrogation. Due to its potential for ‘bad trips,’ ‘flashbacks,’ and potential risk for brain injury, LSD was banned federally in 1966 and is currently only used illicitly. LSD is chemically similar to other naturally occurring lysergamides found in multiple species of morning glory.
Chemical Properties
crystalline solid
History
lysergic acid diethylamide(LSD) is a hallucinogenic drug that was first synthesized a Swiss scientist in the 1930s. During the Cold War, the CIA conducted clandestine experiments with LSD (and other drugs) for mind control, information gathering and other purposes. Over time, the drug became a symbol of the 1960s counterculture, eventually joining other hallucinogenic and recreational drugs at rave parties.
Uses
Potent hallucinogen; non-selective serotonin receptor agonist. Has been used experimentally as adjunct in study and treatment of mental disorders. Controlled substance.
Uses
Labelled Lysergide (L488010)
Uses
LSD is obtained by partial synthesis fromD-lysergic acid. It is also produced bymicrobial reaction of Claviceps paspali overthe hydroxylethylamide. It is a well-knownhallucinogen and a drug of abuse, listed asa controlled substance in the U.S. Code ofFederal Regulations (Title 21, Part 1308.11,1987). It is used as an antagonist to serotoninand in the study and treatment of mentaldisorders.
Definition
ChEBI: An ergoline alkaloid arising from formal condensation of lysergic acid with diethylamine.
General Description
Prismatic crystals (from benzene). Tasteless and odorless. A hallucinogen.
Reactivity Profile
An amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
LSD is a strong psychedelic agent. Theeffects in human are excitement, euphoria,hallucinations, and distorted perceptions.It alters the thinking process, producingillusions and loss of contact with reality.In humans, a dose (intramuscular)of 0.7–0.9 mg/kg or an oral dose of2.5–3.0 mg/kg may produce the effectsabove. Other symptoms may include nausea,vomiting, dilation of pupils, restlessness, andperipheral vascoconstriction. However, thereis no reported case of overdose death. In rabbits,somnolence, ataxia, and an increase inbody temperature were the symptoms notedat the LD50 (intravenous) doses at 0.3 mg/kg.
LD50 value, intravenous (mice): 46 mg/kg
LD50 value, subcutaneous (guinea pigs):16 mg/kg.
Fire Hazard
Flash point data for LSD are not available; however LSD is probably combustible.
Safety Profile
Poison by ingestion, subcutaneous, intraperitoneal, and intravenous routes. Mutation data reported. Human systemic effects by ingestion and intramuscular routes: euphoria, hallucinations, distorted perceptions, excitement, anorexia, nausea and vomiting. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A much-abused hallucinogen. A federally regulated substance. When heated to decomposition it emits toxic fumes of NOx
Environmental Fate
LSD may exist in the air or soil. In the air, LSD may be susceptible to photochemical reactions with subsequently induced radical analogs. Photolysis and oxidative degradation may occur with an airborne half-life of 18 min. LSD’s pKa of 7.8 will have a meaningful percentage of the drug in the cationic form allowing it to interact with soil.
Toxicity evaluation
LSD’s mechanism of action is not completely understood. LSD’s hallucinogenic effects are secondary to its ability to increase central serotonin activity. LSD also stimulates both D1 and D2 dopamine receptors.