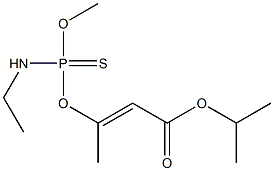
PROPETAMPHOS
- CAS No.
- 31218-83-4
- Chemical Name:
- PROPETAMPHOS
- Synonyms
- Magget;blotic;deadmag;oms1502;san322i;SAN 322;VEL-4283;ent27989;SAFROTIN;(e)-este
- CBNumber:
- CB7478592
- Molecular Formula:
- C10H20NO4PS
- Molecular Weight:
- 281.31
- MDL Number:
- MFCD00137384
- MOL File:
- 31218-83-4.mol
| Melting point | <25℃ |
|---|---|
| Boiling point | bp0.005 87-89° |
| Density | 1.1294 g/cm3 (20 ºC) |
| vapor pressure | 1.9×10-3Pa (20°C) |
| refractive index | nD20 1.495 |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| Water Solubility | 110 mg l-1(24°C) |
| pka | 0.94±0.70(Predicted) |
| Merck | 13,7907 |
| BRN | 1979853 |
| Stability | Light Sensitive, Moisture Sensitive |
| FDA UNII | G4A07F635U |
| Pesticides Freedom of Information Act (FOIA) | Propetamphos |
| EPA Substance Registry System | Propetamphos (31218-83-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301+H311-H330-H410 |
| Precautionary statements | P273-P280-P301+P310+P330-P302+P352+P312-P304+P340+P310 |
| Hazard Codes | T,N |
| Risk Statements | 25-50/53 |
| Safety Statements | 37-45-60-61 |
| RIDADR | 3018 |
| WGK Germany | 3 |
| RTECS | GQ4750000 |
| HazardClass | 6.1(b) |
| PackingGroup | II |
| HS Code | 29299090 |
| Toxicity | LD50 orally in male rats: 82 mg/kg (Berg, Gothe) |
PROPETAMPHOS price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 34371 | Propetamphos PESTANAL | 31218-83-4 | 100mg | $157 | 2024-03-01 | Buy |
| TRC | P769600 | Propetamphos(>85%) | 31218-83-4 | 1g | $605 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 48409 | Propetamphos | 31218-83-4 | 500mg | $610 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PST0000258 | PROPETAMPHOS 95.00% | 31218-83-4 | 5MG | $501.38 | 2021-12-16 | Buy |
| AHH | MT-58191 | Propetamphos 98% | 31218-83-4 | 1g | $410 | 2021-12-16 | Buy |
PROPETAMPHOS Chemical Properties,Uses,Production
Description
Propetamphos technical is a yellowish, oily liquid at room temperature and a moderately toxic acaricide and insecticide. It is sparingly soluble in water, but very soluble in acetone, chloroform, ethanol, and hexane. U.S. EPA has classified propetamphos both as a GUP and RUP, indicating that its handling should be done by certified, qualified, and applicators. Propetamphos is used to control cockroaches, flies, ants, ticks, moths, fleas, and mosquitoes in households and where vector eradication is necessary to protect public health. It is also used in veterinary applications to combat parasites such as ticks, lice, and mites in livestock. The formulations of propetamphos include aerosols, emulsified concentrates, liquids, and powders. In veterinary applications, the insecticide is used for the control of ticks, lice, and mites in livestock. Commercial products include aerosols, emulsified concentrates, liquids, and powders.
Chemical Properties
Propetamphos technical is a yellowish, oily liquid at room temperature and a moderately toxic acaricide and insecticide. It is sparingly soluble in water, but very soluble in acetone, chloroform, ethanol, and hexane. The US EPA has classifi ed propetamphos as a GUP and RUP, indicating that its handling should be done by certifi ed, qualifi ed applicators. Propetamphos is used for the control of cockroaches, fl ies, ants, ticks, moths, fl eas, and mosquitoes in households and where vector eradication is necessary to protect public health. It is also used in veterinary applications to combat parasites, such as ticks, lice, and mites in livestock. The formulations of propetamphos include aerosols, emulsifi ed concentrates, liquids, and powders. Commercial products include aerosols, emulsifi ed concentrates, liquids, and powders.
Uses
Propetamphos is used to control household and public health pests, especially cockroaches, clothes moths and ants. It is also used for the control of ectoparasites (mites, lice and ticks).
Uses
Insecticide.
Uses
Propetamphos may be used as an analytical reference standard for the determination of the analyte in human tissues, fruits and crops by various chromatography techniques.
Definition
ChEBI: Propetamphos is a phosphoramidate ester, an organophosphate insecticide and an isopropyl ester. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor and an agrochemical. It is functionally related to an isopropyl 3-hydroxybut-2-enoate.
General Description
Yellow oily liquid. Non corrosive. Used as an insecticide.
Air & Water Reactions
Water soluble.
Reactivity Profile
An organophosphate derivative. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
Propetamphos is a moderately toxic organophosphate insecticide. It inhibits the cholinesterase enzyme in animals and humans leading to overstimulation of the CNS. Prolonged period of exposures to high concentrations of propetamphos cause poisoning with symptoms that include, but are not limited to, nausea, headache, dizziness, confusion, numbness, tingling sensations, incoordination, tremor, abdominal cramps, sweating, blurred vision, breathing diffi culty, unconsciousness, and convulsions, slow heart beat, respiratory paralysis, and death. There is no evidence to suggest that propetamphos causes mutagenic, teratogenic, or carcinogenic effects in animals or humans.
Agricultural Uses
Fungicide, Insecticide: Propetamphos is used indoors for the control of structural insects, e. g., ants, cockroaches, fleas, and termites. It is applied in indoor residential, medical, commercial, and industrial buildings, and in food service
Trade name
BLOTIC®; OVIDIP®; SAFROTIN®[C]; SAN-52139®; SANDOZ®52139; SERAPHOS®; TSAR®; VEL-4283®; ZOECON®
Safety Profile
Poison by ingestion. Moderately toxic by skin contact. When heated to decomposition it emits very toxic fumes of POx, SOx, and NOx.
Carcinogenicity
There was no significant treatment-
related increase in the incidence of any tumor type of
cancer among male rats given diets with 0, 6, 12, and 120 ppm
(0, 0.376, 0.632, and 5.891 mg/kg/day (males) and, 0, 0.412,
0.689, and 7.602 mg/kg/day (females), respectively) for
24 months . In females, there was an increase in
pancreatic islet cell adenomas at all dose levels compared
to controls. A significant decrease in body weight was
observed in both sexes at the high dose.
When propetamphos was administered to mice via the
diet at dose levels of 0, 0.5, 1.0, 6.0, and 21 mg/kg/day for
91 weeks, there was no apparent increase in the incidence of
tumors in either sex at dose levels that caused significant
cholinesterase inhibition . There was no adverse effect
on survival of males, but high-dose females had a higher
mortality rate than control females and a shorter survival
time. There were no adverse effects on body weight or bodyweight
gain in either sex.
Metabolic pathway
The major pathways of propetamphos detoxification in insect tissue and in the mouse differ, with the principal route in insects being glutathione conjugation but O-demethylation in the mouse. Oxidative or hydrolytic cleavage of the P-O-crotonyl linkage to yield isopropyl acetoacetate, which is hydrolysed and further metabolised to CO2, is also an important route. As with mevinphos, which also contains a crotonyl ester group, de-esterification is not an important detoxification route in animals and no propetamphos carboxylic acid has been detected in the animal experiments which have been reported.
Metabolism
Propetamphos administered in animals is rapidly metabolized and excreted mainly via urine and exhaled air. The major pathways of detoxication in mammals are O-demethylation and cleavage of the P?O-vinyl linkage to give isopropyl acetoacetate, which is finally metabolized to carbon dioxide. Hydrolysis of the carboxylic ester bond is also involved. Activation by oxidative desulfuration also occurs.
Degradation
The hydrolytic DT50, values at pH 3, 6 and 9 were 11 days, 1 year and 41 days respectively. Propetamphos is stable to light and no degradation was detected after 70 hours of exposure to sunlight (PM).
Toxicity evaluation
The acute oral LD50 for rats is 59.5–119 mg/kg. Inhalation LC50 (4 h) for rats is 0.69 (female) and >1.5 (male) mg/L air. NOEL (2 yr) for rats is 6 mg/kg diet (0.3 mg/kg/d).
PROPETAMPHOS Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
View Lastest Price from PROPETAMPHOS manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-25 | PROPETAMPHOS
31218-83-4
|
US $8.00 / kg | 1kg | 99% | 200000 | Hebei Jingbo New Material Technology Co., Ltd |
-

- PROPETAMPHOS
31218-83-4
- US $8.00 / kg
- 99%
- Hebei Jingbo New Material Technology Co., Ltd