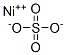Nickel sulfate
- CAS No.
- 7786-81-4
- Chemical Name:
- Nickel sulfate
- Synonyms
- Nickel(II) sulfate;Nickel sulfate 7786-81-4 Nickel(II) sulfate;nci-c60344;Nickel sulfate;nickel(2+)sulfate;NICKELOUSSULPHATE;Nickelous sulfate;nickel(ii)sulphate;nickelsulfate(1:1);nickelsulfate(niso4)
- CBNumber:
- CB7498462
- Molecular Formula:
- NiO4S
- Molecular Weight:
- 154.76
- MDL Number:
- MFCD00011146
- MOL File:
- 7786-81-4.mol
- MSDS File:
- SDS
| Melting point | 848°C |
|---|---|
| Density | 3.68 |
| form | green-yellow orthorhombic crystals |
| color | green-yellow orthorhombic crystals, crystalline |
| Odor | odorless |
| Water Solubility | 27.3-27.7 g/100 mL at 20 ºC |
| CAS DataBase Reference | 7786-81-4(CAS DataBase Reference) |
| FDA UNII | 4FLT4T3WUN |
| EPA Substance Registry System | Nickel sulfate (7786-81-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302+H332-H315-H317-H334-H341-H350i-H360D-H372-H410 |
| Precautionary statements | P202-P273-P280-P301+P312-P302+P352-P308+P313 |
| Hazard Codes | Xn,N,T |
| Risk Statements | 22-40-42/43-50/53-51/53-43-68-48/23-61-49-38-20/22 |
| Safety Statements | 22-36/37-60-61-45-23-53 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | QR9350000 |
| HazardClass | 9 |
| PackingGroup | III |
Nickel sulfate price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 656895 | Nickel(II) sulfate anhydrous, 99.99% trace metals basis | 7786-81-4 | 10g | $79.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 656895 | Nickel(II) sulfate anhydrous, 99.99% trace metals basis | 7786-81-4 | 50g | $284 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | ING0004578 | NICKEL SULPHATE 95.00% | 7786-81-4 | 10G | $1153.33 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | ING0004578 | NICKEL SULPHATE 95.00% | 7786-81-4 | 50G | $2645.61 | 2021-12-16 | Buy |
Nickel sulfate Chemical Properties,Uses,Production
Physical Properties
Nickel sulfate (NiSO4) exists in different states depending on its hydrated forms (where water molecules bond with ions in suspended substances). Nickel sulfate can be in the form of greenish-yellow, blue, or green crystals, depending upon the degree of hydration. It is used in nickel-plating iron and copper, as a catalyst, as a mordant in the textile industry, and as a coating for other substances.
The anhydrous salt is a yellow cubic crystalline solid; density 3.68 g/cm3; decomposes at 848°C; readily dissolves in water; insoluble in ethanol, ether and acetone.
The hexahydrate, NiSO4•6H2O occurs in two crystalline forms, a blue tetragonal alpha-allotrope and an emerald green monoclinic beta form. The blue alpha form converts to green beta above 53.3°C. The beta form is isomorphous with magnesium sulfate hexahydrate, MgSO4•6H2O. Density of hexahydrate is 2.07 g/cm3; refractive index 1.511; loses water on heating; very soluble in water; also very soluble in ethanol and ammonia solution.
The heptahydrate, NiSO4•7H2O, forms green rhombohedral crystals; refractive index 1.467; density 1.948 g/cm3; loses water on heating; highly soluble in water (about 75 g/100mL at 15°C); soluble in alcohol.

nickel sulfate crystals
Uses
Nickel Sulfate is used to make other Nickel compounds, as a mordant, in dyeing and printing textiles, in nickel plating, in coloring metals, in ceramics and for producing driers for use in protective coatings.
Preparation
Nickel sulfate can be made by several methods. It is prepared by dissolving nickel metal, its oxide, or its carbonate in sulfuric acid. In such methods, powdered metal or black nickel oxide is added to hot dilute sulfuric acid, or nickel carbonate is added to dilute sulfuric acid at ambient temperature:
NiO + H2SO4 → NiSO4 + H2O
NiCO3 + H2SO4 → NiSO4 + CO2 + H2O
Impurities may be precipitated by treating the diluted solution with barium carbonate. Evaporation followed by cooling crystallizes hexahydrate in two modifications: blue tetragonal crystals obtained between 31.5 and 53.3°C, and above 53.3°C green monoclinic crystals form. The heptahydrate, NiSO4•7H2O, crystallizes at ordinary temperatures from pure aqueous solutions.
Nickel sulfate also can be produced in large-scale by gas phase reaction of nickel tetracarbonyl, sulfur dioxide, and oxygen at 100°C:
Ni(CO)4 + SO2 + O2 → NiSO4 + 4CO
Reactions
Hydrated nickel sulfate on heating at 103°C loses all of its water molecules. At 848°C, the anhydrous sulfate decomposes to nickel oxide and sulfur trioxide: NiSO4 → NiO + SO3
Nickel sulfate is used in preparing many insoluble nickel salts. In aqueous solutions, such insoluble nickel compounds precipitate; e.g.,
3Ni2+ + 3PO43¯ → Ni3(PO4)2
Nickel sulfate forms double salts with ammonium or alkali metal sulfates. For example, blue-green hydrated ammonium nickel sulfate, (NH4)2SO4•NiSO4•6H2O, crystallizes from a mixed solution of nickel sulfate and ammonium sulfate. Such double sulfates are isomorphous to corresponding alkali metal or ammonium double sulfates of iron, cobalt, magnesium, zinc, and other bivalent metals.
Description
Nickel sulphate appears as blue to blue-green transparent crystals and is an odourless soluble nickel salt. Nickel sulphate is incompatible with strong acids. Nickel sulphate has extensive industrial applications in nickel patch testing, in nickel plating, as a raw material for the production of catalysts, in dyeing and printing fabrics as a mordant, and for blackening zinc and brass and in jewellery manufacture.
Chemical Properties
Nickel sulfate is a blue to blue-green crystalline solid. It has a sweet taste (do not test).
Chemical Properties
(1) Yellow-green crystals, (2) blue or emerald green crystals, (3) green crystals.All the sulfates are soluble in water; (2) and (3) are soluble in alcohol; (1) is insoluble in alcohol and ether.
Occurrence
Nickel sulfate heptahydrate occurs in nature as the mineral morenosite.Probably, the most important uses of nickel sulfate are as an electrolyte in nickel plating and electrorefining. Nickel sulfate also is used as a mordant in dyeing and printing textiles. Other uses are in the preparation of many nickel compounds and nickel catalysts; as a reducing agent; for imparting nickel coating or flashing on steel surface; and for blackening zinc and brass.
Uses
Nickel sulfate (NiSO4) exists in different states depending on its hydrated forms (where water molecules bond with ions in suspended substances). Nickel sulfate can be in the form of greenish-yellow, blue, or green crystals, depending upon the degree of hydration. It is used in nickel-plating iron and copper, as a catalyst, as a mordant in the textile industry, and as a coating for other substances.
Uses
Manufacture of nickel ammonium sulfate, nickel catalysts, nickel plating, mordant in dyeing and printing textiles, coatings, ceramics.
Definition
ChEBI: A metal sulfate having nickel(2+) as the metal ion.
Nickel sulfate, NiS04, is either yellow-green crystals which are soluble in water and insoluble in alcohol and ether,or blue,emerald-green, or green crystals which are soluble in water and alcohol. Derivation is by action of sulfuric acidonnickel. Used in the manufacture of nickel ammonium sulfate, nickel catalysts, nickel plating, and as a mordant in dyeing and printing textiles, coatings,and ceramics.
General Description
Anhydrous Nickel sulfate is a yellow-green crystalline solid. Nickel sulfate can also be obtained as a hexahydrate (NiSO4.6H2O (CAS: 10101-97-0) which is blue to emerald green, and as a heptahydrate (NiSO4.7H2O) (CAS: 10101-98-1) , which is green. Samples can contain variable quantities of water, depending on their previous exposure to moisture or conditions. All forms are mildly toxic and are carcinogenic. All are denser than water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used to make other nickel compounds, in printing, and in dyeing of textiles.
Air & Water Reactions
Water soluble yielding acidic corrosive water solutions.
Reactivity Profile
Gives an acidic solution when dissolved in water. Emits highly toxic fumes of metallic nickel, oxides of sulfur, and oxides of nitrogen when heated to decomposition [Lewis, 3rd ed., 1993, p. 910].
Hazard
Toxic material. Questionable carcinogen.
Health Hazard
Dermatitis.
Flammability and Explosibility
Non flammable
Industrial uses
Nickel sulfate is the most widely used salt for nickel-plating baths, and is known in the plating industry as single nickel salt. It is easily produced by the reaction of sulfuric acid on nickel, and comes in pea-green water-soluble crystalline pellets of the compositionNiSO4.7H2O, of a specific gravity of 1.98, melting at about 100 C. Double nickel salt is nickel ammonium sulfate,NiSO4.(NH4)2.SO4.6H2O , used especially for plating on zinc. To produce a harder and whiter finish in nickel plating, cobaltous sulfamate, a water-soluble powder of the composition Co(NH2SO3)2.3H2O, is used with the nickel sulfate. Nickel plate has a normal hardness of Brinell 90 to 140, but by controlled processes file-hard plates can be obtained from sulfate baths. In electroless plating, nickel sulfate, a reducing agent, a pH adjuster, and complexing and stabilizing agents are combined to deposit metallic nickel on an immersed object.The electroless nickel coating is comparable to electrolytic chrome.
Safety Profile
Confirmed human carcinogen with experimental tumorigenic data. Poison by intravenous, intraperitoneal, and subcutaneous routes. Human mutation data reported. A human skin irritant. When heated to decomposition it emits very toxic fumes of SOx. See also NICKEL COMPOUNDS and SULFATES.
Potential Exposure
Nickel sulfate is used in plating baths, and as an intermediate in the production of nickel ammonium sulfate; as a mordant in dyeing, and printing textiles; coatings, and ceramics.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. The aqueous solution is a weak acid. Sulfates may react violently with aluminum, magnesium.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Nickel sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Tangshan Moneide Trading Co., Ltd. | +86-315-8309571 +8615633399667 | sales@moneidechem.com | China | 568 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12439 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
Related articles
- The introduction of Nickel sulfate
- Anhydrous nickel sulfate is a yellow-green crystalline solid. Nickel sulfate can also be obtained as a hexahydrate which is bl....
- May 11,2022
View Lastest Price from Nickel sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-07 | Nickel sulfate
7786-81-4
|
US $1.00 / kg | 1kg | Ni:33% | 20tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-03-27 | Nickelsulfate
7786-81-4
|
US $5.00 / KG | 1KG | 0.99 | 5t | XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD | |
 |
2023-01-31 | nickel sulfate
7786-81-4
|
US $5150.00 / T | 1KG | 99% | 20 tons | Hebei Mojin Biotechnology Co., Ltd |
-

- Nickel sulfate
7786-81-4
- US $1.00 / kg
- Ni:33%
- Hebei Yanxi Chemical Co., Ltd.
-

- Nickelsulfate
7786-81-4
- US $5.00 / KG
- 0.99
- XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD
-

- nickel sulfate
7786-81-4
- US $5150.00 / T
- 99%
- Hebei Mojin Biotechnology Co., Ltd