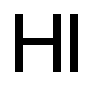Hydriodic acid
- CAS No.
- 10034-85-2
- Chemical Name:
- Hydriodic acid
- Synonyms
- HYDROIODIC ACID;HYDROGEN IODIDE;Hydroiodic Acid 55% to 57% Solution;Hydriodic acid, 57 wt.% solution in H2O;Hydriodic;55-57 wt.%;caswellno482c;Hydrideiodine;hydrogen Hydrogeniodid
- CBNumber:
- CB7852570
- Molecular Formula:
- HI
Lewis structure
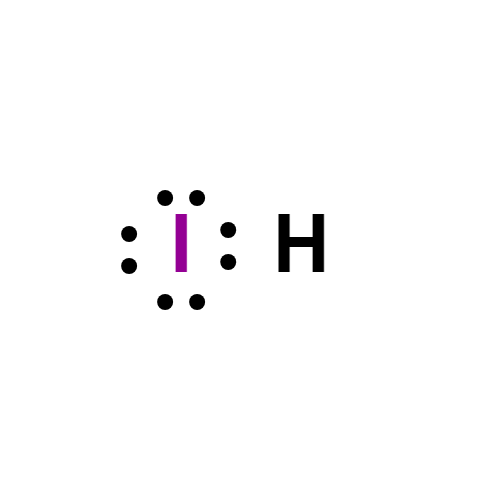
- Molecular Weight:
- 127.91
- MDL Number:
- MFCD00011347
- MOL File:
- 10034-85-2.mol
- MSDS File:
- SDS
| Melting point | -50.8° |
|---|---|
| Boiling point | 127 °C(lit.) |
| Density | 1.96 g/mL at 20 °C |
| vapor pressure | 721.8kPa at 20℃ |
| Flash point | 126-127°C |
| storage temp. | 2-8°C |
| solubility | very soluble in H2O; soluble in organic solvents |
| pka | -10(at 25℃) |
| form | colorless or yellow gas |
| color | Colorless to brown |
| Odor | Pungent odor |
| PH Range | 1 |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4776 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Dielectric constant | 3.4(-50℃) |
| Stability | Stable. Incompatible with bases, amines. Corrodes steel. May discolour on exposure to air and light. |
| CAS DataBase Reference | 10034-85-2(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | HYDRIODIC ACID |
| EWG's Food Scores | 1 |
| FDA UNII | 694C0EFT9Q |
| NIST Chemistry Reference | Hydrogen iodide(10034-85-2) |
| EPA Substance Registry System | Hydriodic acid (10034-85-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H314-H411 | |||||||||
| Precautionary statements | P234-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34-35 | |||||||||
| Safety Statements | 26-36/37/39-45-9 | |||||||||
| RIDADR | UN 1787 8/PG 2 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | MW3760000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28111990 | |||||||||
| NFPA 704 |
|
Hydriodic acid price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 210013 | Hydriodic acid contains no stabilizer, distilled, 57 wt. % in H2O, 99.99% trace metals basis | 10034-85-2 | 50ml | $105 | 2024-03-01 | Buy |
| Sigma-Aldrich | 210013 | Hydriodic acid contains no stabilizer, distilled, 57 wt. % in H2O, 99.99% trace metals basis | 10034-85-2 | 250ml | $350 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.00345 | Hydroiodic acid 67% for analysis EMSURE? | 10034-85-2 | 250ML | $624 | 2024-03-01 | Buy |
| Alfa Aesar | 036484 | Hydriodic acid, ACS, 55-58% | 10034-85-2 | 50ml | $53.4 | 2024-03-01 | Buy |
| Alfa Aesar | 036484 | Hydriodic acid, ACS, 55-58% | 10034-85-2 | *4x500ml | $1036 | 2023-06-20 | Buy |
Hydriodic acid Chemical Properties,Uses,Production
Outline
hydriodic acid is a strong acid. Chemical Formula is HI. The molecular weight is 127.91. Colorless gas, pale yellow liquid or blob solid. There is a strong pungent odor. Melting point is-50.8 ℃, the boiling point is-35.38 ℃, relative density is 5.660 (gas), 2.85-4.7 (liquid), the refractive index is 1.46616. And form a white acid mist with water vapor in the air, easily soluble in water and emit a lot of heat to generate Hydriodic acid. Slightly soluble in ethanol. Unstable, heated to decompose into hydrogen and iodine under the light. Significantly decomposition above 300 ℃. Its constant boiling solution is colorless or light yellow fuming liquid, boiling point is 127 ℃, relative density is 1.7015, strong acid (degree of dissociation of 0.1 mol·L-1 hydroiodic solution up to 95%), the solubility of Hydriodic acid in an organic solvent is much smaller than in water, present in non-electrolytes or weak electrolytes, its ionization constant in pyridine is 3 × 10-3. Easily break down into hydrogen and iodine in the air. With a strong reduction, It has the strongest reducibility in hydrohalic acids, may be oxidated to free iodine by Cl2, Br2, concentrated sulfuric acid. Oxidation by air at room temperature, can be oxidated by concentrated nitric acid, concentrated sulfuric acid. It reacts with most metals to form the corresponding iodide and hydrogen. Method: by heating reaction of sodium iodide and phosphoric acid or reaction of phosphorus with iodine, water was added dropwise on a mixture of phosphorus and iodine, can also be derived by suspending iodine in water and ventilation with hydrogen sulfide. Its constant boiling solution is derived by ventilating Hydriodic acid gas into the water. Uses: its constant boiling solution is often used as a reducing agent, a disinfectant, analytical reagents, preparation of iodized salt, synthetic drugs, dyes, perfumes and so on. It is synthesized by iodine vapor and hydrogen under platinum catalytic conditions or derived by hydrolysis of phosphorus triiodide.
The above information is edited by Yan Yanyong of Chemicalbook.
Chemical Properties
Hydroiodic acid is aqueous solution of Hydriodic acid. When fresh, it is colorless, soon precipitates iodine with yellow to brown in air and sunlight. Azeotrope (56.9% HI), the relative density is 1.70. Boiling point is 127 ℃. Goods hydroiodic are divided into three types: 57% HI, relative density is 1.70. 47% HI, relative density is 1.5. 1% HI, relative density is 1.1. Add 1.5% hypophosphorous acid to make it bleach. The critical temperature is 150 ℃: the critical pressure is 8.3MPa. The dielectric constant is 3.57 at-45 ℃. Conductivity is 8.5 × 10-10S · cm-1. Heat of vaporization is 19.76 Kj/mol, melting heat is 2.87 Kj/mol. Easily soluble in water, soluble in organic solvents.

Hydriodic acid is used as a catalyst in the manufacture of acetic acid, as a pharmaceutical intermediate, in various disinfectant/sanitization formulations including teat dips for mastitis control, and in the preparation of organic and inorganic iodides.
Toxicity
It is highly corrosive to most metals, Non-combustible. But it reacts violently with fluorine, potassium nitrate, potassium chlorate and so on. As hydrochloric acid, it has a strong irritation, gases or vapors can irritate the eyes and respiratory system. Liquid can burn the skin. The patient who inhaled vapors should immediately keep away from the contaminated area, put to rest and keep warm. Accidentally splashed into the eyes, rinse immediately with plenty of water for 15min. Contact with skin, wash immediately with plenty of water. Swallowed immediately rinse mouth, rushed to the hospital for treatment.
Production method
Slowly add iodine and red phosphorus to a reactor filled with water, and react under stirring, filter the reaction solution, and distill the filtrate, collect fractions of 125~130 ℃, obtain Hydroiodic.
2P + 5I2 → 2PI5
PI5 + 4H2O → 5HI + H3PO4
Uses
1. Used for producing organic iodide. As general reagents and pharmaceutical intermediates.
2. Used as the analysis reagents, also used in the preparation of the iodide.
3. Used as a reducing agent, also used in the synthesis of alkyl iodine and other alkyl iodide.
4. Used for Determination of methoxy, ethoxy and selenium, dissolution of acid-insoluble inorganic substance, such as an alkaline earth metal sulfate and mercury iodide and so on. Used as a reducing agent. Used for the preparation of iodide.
5. Used for Determination of methoxy, dissolution of acid-insoluble (especially hot) inorganic substance, such as an alkaline earth metal sulfate and mercury iodide and so on. Used as a reducing agent.
Category
Compressed gas and liquefied gas.
Explosive hazardous characteristics
Contacting with alkali metals can be explosive, thermal decomposition into toxic iodine vapor in the case of heat, produce toxic hydroiodic in the case of water.
Flammability hazard characteristics
Combustible in case of N,N-dinitroso pentamethylene tetramine, release hydrogen cyanide gas in case of cyanide toxic, thermal decomposition of toxic iodide gases.
Storage Characteristics
Treasury ventilation, low-temperature drying and stored separately from cyanide, N,N-dinitroso pentamethylene tetramine, bases.
Extinguishing agent
Water
Description
‘Iodine’ is derived from iodes, a Greek word meaning violet. It is a member of the halide family and hydrogen iodide is considered a strong acid.
Chemical Properties
Hydrogen iodide is a colourless to yellow/brown with an acrid odour non-flammable gas. Hydrogen iodide is incompatible with water and other halides. Hydrogen iodide, upon contact with moisture in air, releases dense vapours. Hydrogen iodide reacts with water to form corrosive acids and reacts violently with alkalis. Most metals corrode rapidly on contact with wet hydrogen iodide, and prolonged exposure of hydrogen iodide to fire or intense heat has been reported to cause the container to rupture and rocket.
Physical properties
This is a strong acid, made by dissolving HI gas in
water. However, hydrogen iodide and hydroiodic acid
differ in that the former is a gas under standard conditions
whereas the other is an aqueous solution of said
gas. They are noninterconvertible. That is, once the
acid is formed with water, it cannot be recovered like
HCl or HBr. Hydroiodic acid is used in organic and inorganic
synthesis as one of the primary sources of iodine
and as a reducing agent.
With moist air, HI gas gives a mist (or fumes) of
hydroiodic acid. It is exceptionally soluble in water.
One liter of water will dissolve 425 L of HI, the final
solution containing only four water molecules per molecule
of HI. As stated, although chemically related,
hydroiodic acid is not pure HI but a mixture containing it. Commercial “concentrated” hydroiodic acid usually
contains 90–98% HI by mass.
Uses
Reducing agent, manufacture of inorganic iodides, pharmaceuticals, disinfectants. The 57% acid is also used for analytical purposes, such as methoxyl determinations.
Uses
Hydriodic acid is used in the manufactureof iodides, as a reducing agent, and indisinfectants and pharmaceuticals.
Uses
Hydriodic acid (HI) is a colorless solution formed when hydrogen iodide gas is dissolved in water, commercially of strength 10% HI, frequently colored brown by iodine. There is a maximum constant boiling point 127 °C (774 mm) at 57% HI (distillate) for mixtures of hydriodic acid and water. Hydriodic acid is used in the preparation of iodides, and as an important reagent in organic chemistry.
Definition
hydrogen iodide: A colourless gas,HI; m.p. –51°C; b.p. –35.38°C. It canbe made by direct combination ofthe elements using a platinum catalyst.It is a strong acid dissociating extensivelyin solution (hydroiodic acid or hydriodic acid). It is also a reducingagent.
Preparation
Hydrogen iodide is prepared by direct combination of hydrogen and iodinevapor in the presence of platinum catalyst:
H2 + I2 → 2HI
The compound is produced in commercial scale by reaction of iodine withhydrazine or hydrogen sulfide:
2I2 + N2H4 → 4HI + N2
I2 + H2S → 2HI + S
Hydriodic acid may be prepared by dissolving hydrogen iodide gas in water.The acid also may be obtained by electrolysis of iodine solution or by passinghydrogen sulfide into a suspension of iodine in water and boiling to expelexcess sulfide. After boiling, the precipitated sulfur is removed by filtrationthrough fritted glass plate or glass wool.
Hydriodic acid in small quantities may be prepared by adding water care-fully to a solid mixture of red phosphorus and iodine.
Technical grade hydriodic acid is a 47% HI solution and usually has abrown color due to the presence of free iodine, produced by air oxidation of HI.Hydriodic acid should be stored in the dark to prevent photochemical decom-position, and free from air to prevent oxidation. The addition of 1.5%hypophosphorus acid (H3PO2) prevents oxidative decomposition.
Hydriodic acid also is commercially sold at 57% (azeotropic concentration)and 10% aqueous solutions.
General Description
A colorless to yellow liquid with a pungent odor. Consists of a solution of hydrogen iodide in water. Fumes irritate the eyes and mucous membranes. Corrosive to metals and to tissue.
It is prepared by the reaction of iodine and hydrosulfuric acid or by the reaction of phosphorus plus iodine plus water followed by distillation. Concentrated hydroiodic acid reacts with the oxygen of the air to form free iodine, which gives a brownish color to the solution. It also gives an idea of the reducing nature of this acid. It is an important reagent in organic chemistry and is used commercially in the preparation of iodides.
Air & Water Reactions
Soluble in water with release of heat.
Reactivity Profile
HYDROIODIC ACID reacts exothermically with organic bases (amines, amides) and inorganic bases (oxides and hydroxides of metals). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate [Lewis]. Mixtures with concentrated sulfuric acid can evolve toxic hydrogen iodide gas at a dangerous rate. Decomposes at high temperatures to emit toxic products. Reacts with fluorine, dinitrogen trioxide, nitrogen dioxide/dinitrogen tetraoxide, and fuming nitric acid.
Hazard
Strong irritant. Poison.
Health Hazard
Hydriodic acid is a corrosive liquid thatcan produce burns on contact with the skin.Contact of acid with the eyes can causesevere irritation. The gas, hydrogen iodide, isa strong irritant to the eyes, skin, and mucousmembranes. No exposure limit has been setfor this gas.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Not classified
Potential Exposure
Hydriodic acid is used as a disinfec-tant, an analytical reagent, raw material for pharmaceuti-cals, and to make iodine salts.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-calattention. If victim is conscious, administer water ormilk. Do not induce vomiting.
storage
(1) Color Code- White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location. (2)Color Code- Yellow Stripe (strong reducing agent):Reactivity Hazard; Store separately in an area isolated fromflammables, combustibles, or other yellow-coded materials.Prior toworking with this chemical you should be trainedon its proper handling and storage. Hydriodic acid must bestored to avoid contact with strong acids (such as hydro-chloric, sulfuric, and nitric), chemically active metals (suchas potassium, sodium, magnesium, and zinc), and strongoxidizers (such as chlorine, bromine, and fluorine) sinceviolent reactions occur. Store in tightly closed containers ina cool, well-ventilated area away from heat and moisture.Protectstoragecontainersfromphysicaldamage.Procedures for the handling, use, and storage of cylindersshould be incompliancewith OSHA 1910.101and1910.169, as with the recommendations of the CompressedGas Association.
Shipping
Hydriodic acid solution requires a shipping labelof“CORROSIVE.”It falls in Hazard Class 8 and PackingGroup HI.Hydrogen iodide, anhydrous, requires a shipping label of“POISON GAS.”It falls in Hazard Class 2.3. It is a viola-tion of transportation regulations to refill compressed gascylinders without the express written permission of the :owner.
Purification Methods
Iodine can be removed from aqueous HI, probably as the amine hydrogen triiodide, by three successive extractions using a 4% solution of Amberlite LA-2 (a long-chain aliphatic amine) in CCl4, toluene or pet ether (10mL per 100mL of acid). [Davidson & Jameson Chem Ind (London) 1686 1963.] Extraction with tributyl phosphate in CHCl3 or other organic solvents is also suitable. Alternatively, a De-acidite FF anion-exchange resin column in the OH--form using 2M NaOH, then into its I--form by passing dilute KI solution through, can be used. Passage of an HI solution under CO2 through such a column removes polyiodide. The column can be regenerated with NaOH. [Irving & Wilson Chem Ind (London) 653 1964]. The earlier method was to reflux with red phosphorus and distil in a stream of N2. The colourless product is stored in ampoules in the dark [Bradbury J Am Chem Soc 74 2709 1952, Heisig & Frykholm Inorg Synth I 157 1939]. It fumes in moist air. HARMFUL VAPOURS.
Toxicity evaluation
Hydroiodic acid is a strong irritant. When used as an expectorant, hydroiodic acid is believed to act by irritating the gastric mucosa, which then stimulates respiratory tract secretion.
Incompatibilities
Contact with water forms toxic and cor-rosive fumes. A strong reducing agent. V iolent actions withstrong acids, chemically active metals, magnesium, phos-phorus, perchloric acid, strong oxidizers. Explodeson contact with ethyl hydroperoxide.Protect from moisture,heat, and shock.
Hydriodic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| zhangjiagang vinsce bio-pharm. limited. | 86-512-58389969 | sales1@swellxin.com | CHINA | 111 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12457 | 58 |
| Hebei Binshare New Material Co. Ltd | +8618633865755 | china01@hbbinshare.com | CHINA | 969 | 58 |
View Lastest Price from Hydriodic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-10-08 | Hydriodic acid
10034-85-2
|
US $30.00 / L | 1L | 99% | 20T | Firsky International Trade (Wuhan) Co., Ltd | |
 |
2023-09-25 | Hydrogen iodide
10034-85-2
|
US $80.00 / kg | 1kg | 99% | 50 kg | Anhui Yisheng Technology Co., LTD | |
 |
2023-08-16 | Hydriodic Acid
10034-85-2
|
US $50.00 / KG | 1KG | >99% | 20tons | Weijer International Trade (Hebei) Co., Ltd |
-

- Hydriodic acid
10034-85-2
- US $30.00 / L
- 99%
- Firsky International Trade (Wuhan) Co., Ltd
-

- Hydrogen iodide
10034-85-2
- US $80.00 / kg
- 99%
- Anhui Yisheng Technology Co., LTD
-

- Hydriodic Acid
10034-85-2
- US $50.00 / KG
- >99%
- Weijer International Trade (Hebei) Co., Ltd
10034-85-2(Hydriodic acid)Related Search:
1of4