Methanol
- CAS No.
- 67-56-1
- Chemical Name:
- Methanol
- Synonyms
- jiachun;Methyl alcohol;CH3OH;Carbinol;metanol;ACID RED;Methanol dried;METHYL RED INDICATOR;METHYL RED MIXED SOLUTION;METHYL RED, SPIRIT SOLUBLE
- CBNumber:
- CB7854099
- Molecular Formula:
- CH4O
Lewis structure
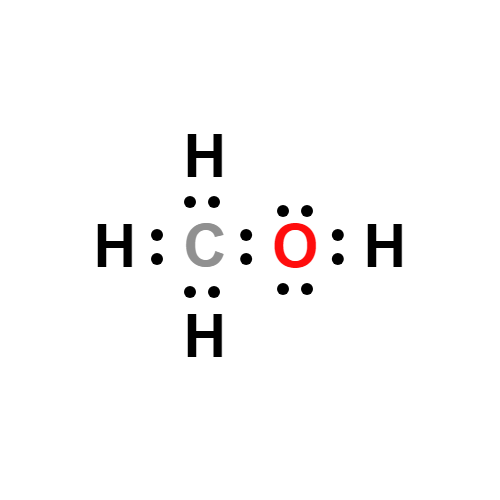
- Molecular Weight:
- 32.04
- MDL Number:
- MFCD00004595
- MOL File:
- 67-56-1.mol
- MSDS File:
- SDS
| Melting point | -98 °C(lit.) |
|---|---|
| Boiling point | 65.4 °C(lit.) |
| Density | 0.791 g/mL at 25 °C |
| vapor density | 1.11 (vs air) |
| vapor pressure | 410 mm Hg ( 50 °C) |
| refractive index |
n |
| Flash point | 52 °F |
| storage temp. | 2-8°C |
| solubility | benzene: miscible(lit.) |
| pka | 15.2(at 25℃) |
| form | Liquid Free From Particulates |
| Specific Gravity | 0.793 (20/20℃) |
| color | <10(APHA) |
| Relative polarity | 0.762 |
| Odor | Faint alcohol odor detectable at 4 to 6000 ppm (mean = 160 ppm) |
| explosive limit | 5.5-44%(V) |
| Odor Threshold | 33ppm |
| Relative density, gas (air=1) | 1.1 |
| Water Solubility | miscible |
| λmax |
λ: 210 nm Amax: 0.50 λ: 220 nm Amax: 0.30 λ: 230 nm Amax: 0.15 λ: 235 nm Amax: 0.10 λ: 240 nm Amax: 0.05 λ: 260 nm Amax: 0.01 λ: 400 nm Amax: 0.01 |
| Merck | 14,5957 |
| BRN | 1098229 |
| Henry's Law Constant | 4.99 at 25 °C (headspace-GC, Gupta et al., 2000) |
| Exposure limits | TLV-TWA (200 ppm) (ACGIH), 260mg/m3, 1040mg/m3 (800 ppm) 15minutes (NIOSH); STEL 310mg/m3 (250 ppm); IDLH 25,000 ppm (NIOSH). |
| Dielectric constant | 33.6(20℃) |
| LogP | -0.770 |
| Substances Added to Food (formerly EAFUS) | METHYL ALCOHOL |
| FDA 21 CFR | 175.105; 175.300; 176.180; 176.200; 177.2460; 177.2800; 73.345; 73.615 |
| CAS DataBase Reference | 67-56-1(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
| NCI Dictionary of Cancer Terms | methanol; methyl alcohol |
| FDA UNII | Y4S76JWI15 |
| Proposition 65 List | Methanol |
| NIST Chemistry Reference | Methyl alcohol(67-56-1) |
| EPA Substance Registry System | Methanol (67-56-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H301+H311+H331-H370 | |||||||||
| Precautionary statements | P210-P280-P301+P310+P330-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 10-20/21/22-68/20/21/22-39/23/24/25-23/24/25-11-40-36-36/38-23/25 | |||||||||
| Safety Statements | 36/37-7-45-16-24/25-23-24-26 | |||||||||
| RIDADR | UN 1170 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | PC1400000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 385 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2905 11 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 oral (rat) 5628 mg/kg LD50 skin (rabbit) 15,840 mg/kg LC50 inhal (rat) >145,000 ppm (1 h) PEL (OSHA) 200 ppm (260 mg/m3) TLV-TWA (ACGIH) 200 ppm (260 mg/m3)—skin STEL (ACGIH) 250 ppm (328 mg/m3) |
|||||||||
| IDLA | 6,000 ppm | |||||||||
| NFPA 704 |
|
Methanol price More Price(243)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | MX0490 | Methanol histology grade | 67-56-1 | 4L | $99.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | MX0490 | Methanol histology grade | 67-56-1 | 20L | $404 | 2024-03-01 | Buy |
| Sigma-Aldrich | NISTRM8509 | Moisture in methanol, 93 mg/kg NIST? SRM? 8509 | 67-56-1 | 5PKG | $763 | 2024-03-01 | Buy |
| Sigma-Aldrich | MX0490 | Methanol histology grade | 67-56-1 | 200L | $1470 | 2024-03-01 | Buy |
| Sigma-Aldrich | MX0488 | Methanol gradient grade OmniSolv? | 67-56-1 | 1L | $86.2 | 2024-03-01 | Buy |
Methanol Chemical Properties,Uses,Production
Introduction
Methanol is the simplest fatty alcohol. It is a colorless, flammable, irritating liquid with a boiling point of 64.7°C, a melting point of -93.90°C, and a relative density of 0.7913. Soluble in water and most organic solvents. Its severe toxicity can damage the optic nerve. Once swallowed, it can make the eyes blind and even cause death.
Methanol has the general properties of a primary aliphatic alcohol. The three hydrogen atoms on a carbon atom with a hydroxyl group can be oxidized, orderly generating formaldehyde, formic acid, and carbon dioxide. Therefore, it is largely used in the synthesis of formaldehyde. Methanol is easily converted into important organic synthesis intermediates such as methyl carboxylate, methyl chloride and methylamine, and it is also applied as important organic solvents, extraction agents and alcohol denaturants.
Chemical Properties
Methanol is a clear, colorless liquid with a characteristic pungent odor (NTP NIEHS web accessed 2/16/2013). The air odor threshold has been reported as 1500 ppm (approximately 2000mg/m3), much higher than the occupational guidelines.

Methanol (methyl alcohol; wood alcohol) is used extensively as a solvent for lacquers, paints, varnishes, cements, inks, dyes, plastics, and various industrial coatings. Large quantities are used in the production of formaldehyde and other chemical derivatives such as acetic acid, methyl halides and terephthalate, methyl methacrylate, and methylamines. Methanol is also used as a gasoline additive, as a component of lacquer thinners, in antifreeze preparations of the “nonpermanent” type, and in canned heating preparations of jellied alcohol. It is also used in duplicating fluid, in paint removers, and as a cleaning agent. The potential for methanol as a future alternative for gasoline indicates that the use of this chemical will most likely increase rather than decrease. At room temperature, methanol is a colorless liquid with a pungent odor. It is relatively volatile, with a vapor pressure of 96 mmHg and a vapor density of 1.11. It is miscible with water and soluble with other organic solvents. It is found in nature as a fermentation product of wood and as a constituent of some fruits and vegetables.
Uses
Methanol is an important chemical raw material for fine chemicals. Its carbonylation at 3.5 MPa and 180-200° C in the presence of catalyst can produce acetic acid and further produce acetic anhydride. It reacts with syngas to prepare vinyl acetate in the presence of catalyst; reacts with isobutylene to produce tert-butyl methyl ether; prepare dimethyl oxalate through oxidization and carbonylation, and a further hydrogenation to produce ethylene glycol; reacts with toluene under catalyst and simultaneous oxidization to produce phenylethyl alcohol. It can be used as a good solvent, as a pesticide raw material, as an antifreeze agent, as a fuel and fuel additive (this is receiving increasing attention in environmental protection field). It is the main raw material in the preparation of formaldehyde, the raw material in medicine and spices production, a solvent in dyes and paint industries, the raw material in preparation of methanol single cell protein and synthesis of methyl ester.
Methanol poisoning and First Aid Measures
Pathogenesis
First, methanol has a cumulative effect and is oxidized in the body into more toxic formaldehyde and formic acid. Methanol and its oxides directly damage the tissues, causing cerebral edema, meningeal hemorrhage, optic nerve and retinal atrophy, pulmonary congestion and edema, and hepatic and renal turbid swelling.
Second, methanol and its oxides cause blood circulation disorder, coenzyme system obstacles in vivo, resulting in lack of oxygen supply to the brain cortical cells, metabolic disorders, and related neurological and psychiatric symptoms.
Third, methanol oxidation products combine with the iron in the cytochrome oxidase, which inhibits the intracellular oxidation process thus causing metabolic disorders, acidosis along with organic acid accumulation in the body, and nerve cells impair.
Treatment
- Keep away from the methanol dispersion area, excrete methanol from the body.
Antidote: Ethanol is an antidote to methanol poisoning. Ethanol can prevent methanol’s oxidation and promote its emission. Prepare 5% ethanol solution using 10% glucose solution, and drip slowly intravenously. - Maintain electrolyte balance: maintain respiratory and circulatory function, provide with a large number of Vitamin B.
Treatment of acidosis: Administrate timely sodium bicarbonate solution or sodium lactate solution based on blood gas analysis, carbon dioxide binding force measurement and clinical performance. - Prevent cerebral edemas actively, reduce intracranial pressure, improve fundus blood circulation, and prevent optic neuropathy if needed.
- Inject intravenously cytochrome C, polar fluid to restore cytochrome oxidase function.
- Control mental state by applying diazepam, perphenazine and the like.
Reactivities of Methanol
Methanol is the simplest aliphatic alcohol. It contains only one carbon atom. Unlike higher alcohols, it cannot form an olefin through dehydration. However, it can undergo other typical reactions of aliphatic alcohols involving cleavage of a C-H bond or O-H bond and displacement of the -OH group. Table 1 summarizes the reactions of methanol, which are classified in terms of their mechanisms. Examples of the reactions and products are given.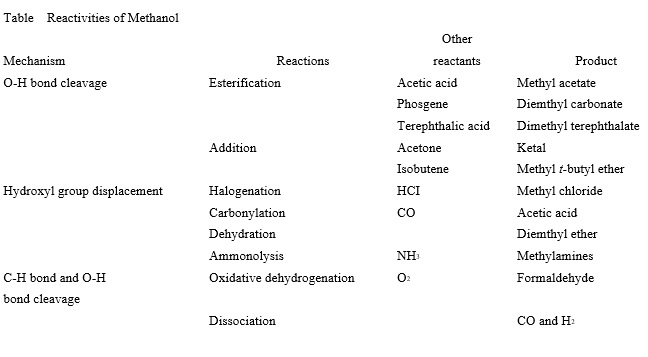
Homolytic dissociation energies of the C-O and O-H bonds in methanol are relatively high. Catalysts are often used to activate the bonds and to increase the selectivity to desired products.
Production
Methanol is prepared by pressure heating with carbon monoxide and hydrogen in the presence of a catalyst:
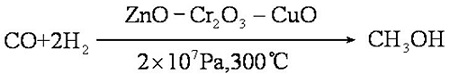
If the conditions are strictly controlled, the yield can reach 100% and the purity can reach 99%. Methane is mixed with oxygen (9:1, V/V) and methanol is obtained through a copper tube under heating and pressure: 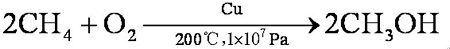
Toxicity evaluation
ADI is limited to GMP (FAO/WHO, 2001).
Toxic, can cause blindness.
LD50: 5628 mg/kg (rat, oral).
Methanol gasoline
Methanol gasoline refers to the M series mixture fuel made of addition of methanol to the gasoline and formulated using methanol fuel solvent. Among them, M15 (add 15% methanol in gasoline) clean methanol gasoline is used as vehicle fuel, respectively, used in a variety of gasoline engines. It can be applied to substitute the finished gasoline without changing the existing engine structure, and can also be mixed with refined oil. The methanol mixed fuel has excellent thermal efficiency, power, start-up and being economical. It is also characterized by lowering the emissions, saving oil and being safe and convenient. Methanol gasoline types of M35, M15, M20, M50, N85 and M100 with different blend ratios have been developed around the world according to the conditions of different countries. At present, the commercial methanol is mainly M85 (85% methanol + 15% gasoline) and M100 with M100 performance being better than M85 and having greater environmental advantages.
Description
Methyl alcohol, also known as methanol or wood alcohol, is a clear, colorless, flammable liquid
that is the simplest alcohol.
World production of methanol is approximately 8.5 billion gallons annually. Methanol
is produced industrially, starting with the production of synthesis gas or syngas. Syngas used
in the production of methyl alcohol is a mixture of carbon monoxide and hydrogen formed
when natural gas reacts with steam or oxygen. Methyl alcohol is then synthesized
from carbon monoxide and hydrogen.
Methyl alcohol is poisonous and is commonly used to denature ethyl alcohol. Methanol
poisoning results from ingestion, inhalation of methanol vapors, or absorption through
the skin. Methanol is transformed in the body to formaldehyde (H2CO) by the enzyme
alcohol dehydrogenase.The formaldehyde is then metabolized to formic acid (HCOOH)by aldehyde dehydrogenase.
Chemical Properties
Methanol is a clear, water-white liquid with a mild odor at ambient temperatures.The air odor threshold for methanol has been reported as 100 ppm . Others have reported that 2000 or 5900 ppm methanol is barely detectable .
Physical properties
Clear, colorless liquid with a characteristic alcoholic odor. Odor threshold concentrations ranged from 8.5 ppbv (Nagata and Takeuchi, 1990) to 100.0 ppmv (Leonardos et al., 1969). Experimentally determined detection and recognition odor threshold concentrations were 5.5 mg/m3 (4.2 ppmv) and 69 mg/m3 (53 ppmv), respectively (Hellman and Small, 1974).
History
It was first isolated in 1661 by the Irish chemist Robert Boyle (1627–1691) who prepared it by the destructive distillation of boxwood, giving it the name spirit of box, and the name wood alcohol is still used for methyl alcohol. Methyl alcohol is also called pyroxylic spirit; pyroxylic is a general term meaning distilled from wood and indicates that methyl alcohol is formed during pyrolysis of wood. The common name was derived in the mid-1800s. The name methyl denotes the single carbon alkane methane in which a hydrogen atom has been removed to give the methyl radical. The word alcohol is derived from Arabic al kuhul.
Uses
high purity grade for ICP-MS detection
Uses
Methanol is used in the production offormaldehyde, acetic acid, methyl tert-butylether, and many chemical intermediates; asan octane improver (in oxinol); and as apossible alternative to diesel fuel; being anexcellent polar solvent, it is widely used as acommon laboratory chemical and as a methylating reagent.
Uses
Methanol has numerous uses. Its main use is in the production of formaldehyde, whichconsumes approximately 40% of methanol supplies. Methanolis a common organic solvent found in many products including deicers (windshield wiperfl uid), antifreezes, correction fl uid, fuel additives, paints, and other coatings. A number ofindustrial chemicals use methanol in their production. Among these are methyl methacrylateand dimethyl terephthalate. Methanol is used to convert methylacrylamide sulfate to methylmethacrylate and ammonium hydrogen sulfate (NH4HSO4):
Methanol is used in making the ester dimethyl terephthalate from mixtures ofxylene of toluene. Dimethyl terephthalate is used in the manufacture of polyesters and plastics.
Methanol is used as a fuel additive. The common gasoline additive HEET is pure methanoland is used as a gas-line antifreeze and water remover. Methanol is used as a fuel in camp stoves and small heating devices. It is used to fuel the small engines used in models (airplanes,boats). In the early history of automobiles,methanol was a common fuel. The availability of cheap gasoline replaced methanol in the1920s, but it is receiving renewed interest as an alternative fuel as the demand and cost of oilincrease and oil supplies become uncertain. Methanol can be produced from coal and biomass.Methanol has a higher octane rating and generally lower pollutant emissions compared togasoline. The relatively low flame temperature means that fewer nitrogen oxides are producedby methanol than by ethanol. One large disadvantage of methanol is that it has a lower energydensity than gasoline. Using equivalent volumes of gasoline and methanol, methanol givesabout half the mileage of gasoline. Another problem with methanol is its low vapor pressure,resulting in starting problems on cold days. This problem can be mitigated by using a blendof 85% methanol and 15% gasoline. This mixture is called M85 and is similar to E85 ethanol(see Ethyl Alcohol).
Uses
Methylalcohol, CH30H, also known as methanol or wood alcohol, is a colorless, toxic, flammable liquid with a boiling point of 64.6 °C(147 °F). The principal toxic effect is on the nervous system,particularly the retinae. Methyl alcoholis miscible in all proportions with water,ethyl alcohol, and ether. It burns with a light blue flame producing water and carbon dioxide. This vapor forms an explosive mixture(6.0 to 36.5% by volume) with air. Methyl alcohol is an important inexpensive raw material that is synthetically produced for the organic chemical industry. Nearly half of the methyl alcohol manufactured is used in the production of formaldehyde. Other uses of methyl alcohol are as an antifreeze and fuel for automobiles and as an intermediate in the production of synthetic protein.
Uses
Industrial solvent. Raw material for making formaldehyde and methyl esters of organic and inorganic acids. Antifreeze for automotive radiators and air brakes; ingredient of gasoline and diesel oil antifreezes. Octane booster in gasoline. As fuel for picnic stoves and soldering torches. Extractant for animal and vegetable oils. To denature ethanol. Softening agent for pyroxylin plastics. Solvent and solvent adjuvant for polymers. Solvent in the manufacture of cholesterol, streptomycin, vitamins, hormones, and other pharmaceuticals.
Production Methods
Modern industrial-scale methanol production is exclusively based on synthesis from pressurized mixtures of hydrogen, carbon monoxide, and carbon dioxide gases in the presence of catalysts. Based on production volume, methanol has become one of the largest commodity chemicals produced in the world.
Definition
ChEBI: Methanol is the primary alcohol that is the simplest aliphatic alcohol, comprising a methyl and an alcohol group. It has a role as an amphiprotic solvent, a fuel, a human metabolite, an Escherichia coli metabolite, a mouse metabolite and a Mycoplasma genitalium metabolite. It is an alkyl alcohol, a one-carbon compound, a volatile organic compound and a primary alcohol. It is a conjugate acid of a methoxide.
Reactions
Methyl alcohol is a versatile material, reacting (1) with sodium metal, forming sodium methylate, sodium methoxide CH3ONa plus hydrogen gas, (2) with phosphorus chloride, bromide, iodide, forming methyl chloride, bromide, iodide, respectively, (3) with H2SO4 concentrated, forming dimethyl ether (CH3)2O, (4) with organic acids, warmed in the presence of H2SO4, forming esters, e.g., methyl acetate CH3COOCH3, [CAS: 79-20-9], methyl salicylate C6H4(OH)·COOCH3, possessing characteristic odors, (5) with magnesium methyl iodide in anhydrous ether (Grignard’s solution), forming methane as in the case of primary alcohols, (6) with calcium chloride, forming a solid addition compound 4CH3OH·CaCl2, which is decomposed by H2O, (7) with oxygen, in the presence of heated smooth copper or silver forming formaldehyde. The density of pure methyl alcohol is 0.792 at 20 °C compared with H2O at 4 °C (the corresponding figure for ethyl alcohol is 0.789), and the percentage of methyl alcohol present in a methyl alcohol-water solution may be determined from the density of the sample.
World Health Organization (WHO)
Methanol has been subjected to abuse by consumption as a substitute for ethanol. Its toxic metabolites cause irreversible blindness and severe metabolic acidosis, and are ultimately fatal. Methanol continues to be used as an industrial solvent.
General Description
A colorless fairly volatile liquid with a faintly sweet pungent odor like that of ethyl alcohol. Completely mixes with water. The vapors are slightly heavier than air and may travel some distance to a source of ignition and flash back. Any accumulation of vapors in confined spaces, such as buildings or sewers, may explode if ignited. Used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products.
Reactivity Profile
Methanol reacts violently with acetyl bromide [Merck 11th ed. 1989]. Mixtures with concentrated sulfuric acid and concentrated hydrogen peroxide can cause explosions. Reacts with hypochlorous acid either in water solution or mixed water/carbon tetrachloride solution to give methyl hypochlorite, which decomposes in the cold and may explode on exposure to sunlight or heat. Gives the same product with chlorine. Can react explosively with isocyanates under basic conditions. The presence of an inert solvent mitigates this reaction [Wischmeyer 1969]. A violent exothermic reaction occurred between methyl alcohol and bromine in a mixing cylinder [MCA Case History 1863. 1972]. A flask of anhydrous lead perchlorate dissolved in Methanol exploded when Methanol was disturbed [J. Am. Chem. Soc. 52:2391. 1930]. P4O6 reacts violently with Methanol. (Thorpe, T. E. et al., J. Chem. Soc., 1890, 57, 569-573). Ethanol or Methanol can ignite on contact with a platinum-black catalyst. (Urben 1794).
Hazard
Flammable, dangerous fire risk. Explosive limits in air 6–36.5% by volume. Toxic by ingestion (causes blindness). Headache, eye damage, dizziness, and nausea.
Health Hazard
The acute toxicity of methanol by ingestion, inhalation, and skin contact is low. Ingestion
of methanol or inhalation of high concentrations can produce headache, drowsiness,
blurred vision, nausea, vomiting, blindness, and death. In humans, 60 to 250 mL is
reported to be a lethal dose. Prolonged or repeated skin contact can cause irritation and
inflammation; methanol can be absorbed through the skin in toxic amounts. Contact of
methanol with the eyes can cause irritation and burns. Methanol is not considered to have
adequate warning properties.
Methanol has not been found to be carcinogenic in humans. Information available is
insufficient to characterize the reproductive hazard presented by methanol. In animal
tests, the compound produced developmental effects only at levels that were maternally
toxic; hence, it is not considered to be a highly significant hazard to the fetus. Tests in
bacterial or mammalian cell cultures demonstrate no mutagenic activity
Health Hazard
Ingestion of adulterated alcoholic beveragescontaining methanol has resulted in innumerable loss of human lives throughout theworld. It is highly toxic, causing acidosis andblindness. The symptoms of poisoning arenausea, abdominal pain, headache, blurredvision, shortness of breath, and dizziness.In the body, methanol oxidizes to formaldehyde and formic acid — the latter could bedetected in the urine, the pH of which is lowered (when poisoning is severe).
The toxicity of methanol is attributed tothe metabolic products above. Ingestion inlarge amounts affects the brain, lungs, gastrointestinal tract, eyes, and respiratory system and can cause coma, blindness, anddeath. The lethal dose is reported to be60–250 mL. The poisoning effect is prolonged and the recovery is slow, often causing permanent loss of sight.
Other exposure routes are inhalation andskin absorption. Exposure to methanol vaporto at 2000 ppm at regular intervals over aperiod of 4 weeks caused upper respiratorytract irritation and mucoid nasal discharge inrats. Such discharge was found to be a doserelated effect.
Inhalation in humans may produce headache, drowsiness, and eye irritation. Prolonged skin contact may cause dermatitis andscaling. Eye contact can cause burns anddamage vision..
Fire Hazard
Behavior in Fire: Containers may explode.
Flammability and Explosibility
Methanol is a flammable liquid (NFPA rating = 3) that burns with an invisible flame in daylight; its vapor can travel a considerable distance to an ignition source and "flash back." Methanol-water mixtures will burn unless very dilute. Carbon dioxide or dry chemical extinguishers should be used for methanol fires.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization:Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
A human poison by ingestion. Poison experimentally by skin contact. Moderately toxic experimentally by intravenous and intraperitoneal routes. Mildly toxic by inhalation. Human systemic effects: changes in circulation, cough, dyspnea, headache, lachrymation, nausea or vomiting, optic nerve neuropathy, respiratory effects, visual field changes. An experimental teratogen. Experimental reproductive effects. An eye and skin irritant. Human mutation data reported. A narcotic. Its main toxic effect is exerted upon the nervous system, particularly the optic nerves and possibly the retinae. The condtion can progress to permanent blindness. Once absorbed, methanol is only very slowly eliminated. Coma resulting from massive exposures may last as long as 2-4 days. In the body, the products formed by its oxidation are formaldehyde and formic acid, both of which are toxic. Because of the slow elimination, methanol should be regarded as a cumulative poison. Though single exposures to fumes may cause no harmful effect, daily exposure may result in the accumulation of sufficient methanol in the body to cause illness. Death from ingestion of less than 30 mL has been reported. A common air contaminant. Flammable liquid. Dangerous fire hazard when exposed to heat, flame, or oxidlzers. Explosive in the form of vapor when exposed to heat or flame. Explosive reaction with chloroform + sodium methoxide, diethyl zinc. Violent reaction with alkyl aluminum salts, acetyl bromide, chloroform + sodlum hydroxide, CrO3, cyanuric chloride, (I + ethanol + HgO), Pb(ClO4)2, HClO4, P2O3, (KOH + CHCb), nitric acid. Incompatible with berylhum dihydride, metals (e.g., potassium, magnesium), oxidants (e.g., barium perchlorate, bromine, sodium hypochlorite, chlorine, hydrogen peroxide), potassium tert-butoxide, carbon tetrachloride + metals (e.g., aluminum, magnesium, zinc), dlchloromethane. Dangerous; can react vigorously with oxidizing materials. To fight fire, use alcohol foam. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Drug,Mutagen; Reproductive Effector; Human Data; PrimaryIrritant. Methyl alcohol is used as a starting material inorganic synthesis of chemicals, such as formaldehyde,methacrylates, methyl amines, methyl halides, ethylene glycol, and pesticides, and as an industrial solvent for inks, resins, adhesives, and dyes. It is an ingredient in paint and varnish removers, cleaning and dewaxing preparations, spirit duplicating fluids, embalming fluids, antifreeze mixtures, and enamels, and is used in the manufacture of photographic film, plastics, celluloid, textile soaps, wood stains, coated fabrics, shatterproof glass, paper coating, waterproofing formulations, artificial leather, and synthetic indigo and other dyes. It has also been used as an extractant in many other processes, an antidetonant fuel-injection fluid for aircraft, a rubber accelerator, and a denaturant for ethyl alcohol.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Source
Methanol occurs naturally in small-flowered oregano (5 to 45 ppm) (Baser et al., 1991),
Guveyoto shoots (700 ppb) (Baser et al., 1992), orange juice (0.8 to 80 ppm), onion bulbs,
pineapples, black currant, spearmint, apples, jimsonweed leaves, soybean plants, wild parsnip,
blackwood, soursop, cauliflower, caraway, petitgrain, bay leaves, tomatoes, parsley leaves, and
geraniums (Duke, 1992).
Methanol may enter the environment from methanol spills because it is used in formaldehyde
solutions to prevent polymerization (Worthing and Hance, 1991).
Environmental Fate
Biological. In a 5-d experiment, [14C]methanol applied to soil water suspensions under aerobic
and anaerobic conditions gave 14CO2 yields of 53.4 and 46.3%, respectively (Scheunert et al.,
1987). Heukelekian and Rand (1955) reported a 5-d BOD value of 0.85 g/g which is 56.7% of the
ThOD value of 1.50 g/g. Using the BOD technique to measure biodegradation, the mean 5-d BOD
value (mM BOD/mM methanol) and ThOD were 0.93 and 62.0%, respectively (Vaishnav et al.,
1987).
Photolytic. Photooxidation of methanol in an oxygen-rich atmosphere (20%) in the presence of
chlorine atoms yielded formaldehyde and hydroxyperoxyl radicals. The reaction is initiated via
hydrogen abstraction by OH radicals or chlorine atoms yielding a hydroxymethyl radical.
Chlorine, formaldehyde, carbon monoxide, hydrogen peroxide, and formic acid were detected
(Whitbeck, 1983). Reported rate constants for the reaction of methanol and OH radicals in the
atmosphere: 5.7 x 10-11 cm3/mol·sec at 300 K (Hendry and Kenley, 1979), 5.7 x 10-8 L/mol·sec
(second-order) at 292 K (Campbell et al., 1976), 1.00 x 10-12 cm3/molecule·sec at 292 K (Meier et
al., 1985), 7.6 x 10-13 cm3/molecule·sec at 298 K (Ravishankara and Davis, 1978), 6.61 x 10-13
cm3/molecule·sec at room temperature (Wallington et al., 1988a). Based on an atmospheric OH
concentration of 1.0 x 106 molecule/cm3, the reported half-life of methanol is 8.6 d (Grosjean,
1997).
Chemical/Physical. In a smog chamber, methanol reacted with nitrogen dioxide to give methyl
nitrite and nitric acid (Takagi et al., 1986). The formation of these products was facilitated when
this experiment was accompanied by UV light (Akimoto and Takagi, 1986).
Methanol will not hydrolyze because it does not have a hydrolyzable functional group (Kollig,
1993).
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 964 mg/L. The adsorbability of the carbon used was 7 mg/g carbon (Guisti et al.,
1974).
Hydroxyl radicals react with methanol in aqueous solution at a reaction rate of 1.60 x 10-12
cm3/molecule?sec (Wallington et al., 1988).
Complete combustion in air produces carbon dioxide and water. The stoichiometric equation for
this oxidation reaction is:
2CH4O + 3O2 → 2CO2 + 4H2O
storage
Methanol should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Shipping
UN1230 Methanol, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous material. (International)
Purification Methods
Almost all methanol is now obtained synthetically. Likely impurities are water, acetone, formaldehyde, ethanol, methyl formate and traces of dimethyl ether, methylal, methyl acetate, acetaldehyde, carbon dioxide and ammonia. Most of the water (down to about 0.01%) can be removed by fractional distillation. Drying with CaO is unnecessary and wasteful. Anhydrous methanol can be obtained from "absolute" material by passage through Linde type 4A molecular sieves, or by drying with CaH2, CaSO4, or with just a little more sodium than required to react with the water present, in all cases the methanol is then distilled. Two treatments with sodium reduces the water content to about 5 x 10-5%. [Friedman et al. J Am Chem Soc 83 4050 1961.] Lund and Bjerrum [Chem Ber 64 210 1931] warmed clean dry magnesium turnings (5g) and iodine (0.5g) with 50-75mL of "absolute" methanol in a flask until the iodine disappeared and all the magnesium was converted to the methoxide. Up to 1L of methanol was added and, after refluxing for 2-3hours, it was distilled off, excluding moisture from the system. Redistillation from tribromobenzoic acid removes basic impurities and traces of magnesium oxides, and leaves conductivity-quality material. The method of Hartley and Raikes [J Chem Soc 127 524 1925] gives a slightly better product. This consists of an initial fractional distillation, followed by distillation from aluminium methoxide, and then ammonia and other volatile impurities are removed by refluxing for 6hours with freshly dehydrated CuSO4 (2g/L) while dry air is passed through: the methanol is finally distilled. (The aluminium methoxide is prepared by warming with aluminium amalgam (3g/L) until all the aluminium has reacted. The amalgam is obtained by warming pieces of sheet aluminium with a solution of HgCl2 in dry methanol.) This treatment also removes aldehydes. If acetone is present in the methanol, it is usually removed prior to drying. Bates, Mullaly and Hartley [J Chem Soc 401 1923] dissolved 25g of iodine in 1L of methanol and then poured the solution, with constant stirring, into 500mL of M NaOH. Addition of 150mL of water precipitated iodoform. The solution was allowed to stand overnight, filtered, then boiled under reflux until the odour of iodoform disappeared, and fractionally distilled. (This treatment also removes formaldehyde.) Morton and Mark [Ind Eng Chem (Anal Edn) 6 151 1934] refluxed methanol (1L) with furfural (50mL) and 10% NaOH solution (120mL) for 6-12hours, the refluxing resin carries down with it the acetone and other carbonyl-containing impurities. The alcohol was then fractionally distilled. Evers and Knox [J Am Chem Soc 73 1739 1951], after refluxing 4.5L of methanol for 24hours with 50g of magnesium, distilled off 4L of it, which they then refluxed with AgNO3 for 24hours in the absence of moisture or CO2. The methanol was again distilled, shaken for 24hours with activated alumina before being filtered through a glass sinter and distilled under nitrogen in an all-glass still. Material suitable for conductivity work was obtained. Variations of the above methods have also been used. For example, a sodium hydroxide solution containing iodine has been added to methanol and, after standing for 1day, the solution has been poured slowly into about a quarter of its volume of 10% AgNO3, shaken for several hours, then distilled. Sulfanilic acid has been used instead of tribromobenzoic acid in Lund and Bjerrum's method. A solution of 15g of magnesium in 500mL of methanol has been heated under reflux, under nitrogen, with hydroquinone (30g), before degassing and distilling the methanol, which was subsequently stored with magnesium (2g) and hydroquinone (4g per 100mL). Refluxing for about 12hours removes the bulk of the formaldehyde from methanol: further purification has been obtained by subsequent distillation, refluxing for 12hours with dinitrophenylhydrazine (5g) and H2SO4 (2g/L), and again fractionally distilling. [Beilstein 1 IV 1227.]
Toxicity evaluation
The toxic properties of methanol are the result of accumulation
of the formate intermediate in the blood and tissues of exposed
individuals. Formate accumulation produces metabolic
acidosis leading to the characteristic ocular toxicity (blindness)
observed in human methanol poisonings.
Humans and primates appear particularly sensitive to
methanol toxicity when compared to rats. This is attributed to
the slower rate of conversion in humans of the formate
metabolite via tetrahydrofolate. This step in methanol metabolism
occurs in rats at a rate ~2.5 times that observed in
humans.
Formate appears to directly affect the retina and optic nerve
by acting as a mitochondrial toxin. It is believed that formate
acts as a metabolic poison by inhibiting cytochrome oxidase
activity. The cells of the optic nerve have low reserves of cytochrome
oxidase and thus may be particularly sensitive to
formate-induced metabolic inhibition.
Incompatibilities
Methanol reacts violently with strong oxidizers, causing a fire and explosion hazard.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration
Methanol Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15745 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| ARCTIC EXPORTS INC | +1-3026880818 +1-3026880818 | ARCTICEXPORTSINC@GMAIL.COM | Canada | 68 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
| Hunan aslsen technology co.,ltd | +86-15308460054 +86-19313031929 | aslsd@aslsen.com | China | 128 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
Related articles
- Why is methanol a polar molecule?
- Methanol, its molecular FormulaCH3OH, is a flammable, colorless, and volatile liquid with a pronounced alcoholic odor.
- Dec 21,2023
- Methanol:General description,Application,and Production
- Methanol, also known as methyl alcohol, wood alcohol, or carbinol, is a clear, colorless liquid with a slightly sweet odor.
- Apr 28,2023
- METHANOL:Commercially Important Alcohols
- Methanol (methyl alcohol) was originally produced by heating wood chips in the absence of air. Some of the carbohydrates in th....
- Nov 29,2019
View Lastest Price from Methanol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-25 | Methanol
67-56-1
|
US $1.00 / g | 1g | 99 | 20tons | Shanghai Longyu Biotechnology Co., Ltd. | |
 |
2024-04-24 | Methanol
67-56-1
|
US $693.00-685.00 / metric tonnes | 1metric tonnes | 99% | 1000tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-23 | Methyl alcohol
67-56-1
|
US $10.00 / kg | 1kg | 99% | 100 tons | Hunan aslsen technology co.,ltd |
-

- Methanol
67-56-1
- US $1.00 / g
- 99
- Shanghai Longyu Biotechnology Co., Ltd.
-

- Methanol
67-56-1
- US $693.00-685.00 / metric tonnes
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Methyl alcohol
67-56-1
- US $10.00 / kg
- 99%
- Hunan aslsen technology co.,ltd







