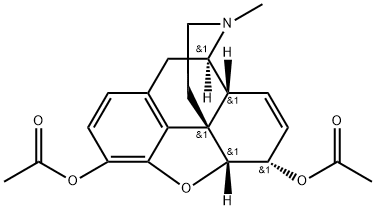
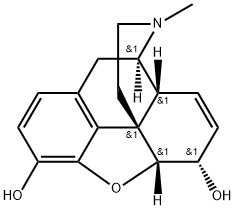
HEROIN
- CAS No.
- 561-27-3
- Chemical Name:
- HEROIN
- Synonyms
- Diamorphine;BOY;DIACETYLMORPHINE;diacetylmorphone;dava;junk;Horse;rufus;smack;Heroine
- CBNumber:
- CB8243173
- Molecular Formula:
- C21H23NO5
- Molecular Weight:
- 369.42
- MDL Number:
- MFCD00468072
- MOL File:
- 561-27-3.mol
| Melting point | 154-161°C |
|---|---|
| alpha | D25 -166° (c = 1.49 in methanol) |
| Boiling point | bp12 272-274° |
| Density | 1.5600 |
| refractive index | 1.5614 (estimate) |
| Flash point | 2℃ |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | H2O: <0.2 mg/mL |
| form | A neat solid |
| pka | pKa 7.83 (Uncertain) |
| NCI Dictionary of Cancer Terms | heroin |
| FDA UNII | 70D95007SX |
| ATC code | N07BC06 |
| EPA Substance Registry System | Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-methyl-(5.alpha.,6.alpha.)-, 3,6-diacetate (561-27-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H312-H319-H331 | |||||||||
| Precautionary statements | P210-P261-P302+P352+P312-P304+P340+P312-P337+P313-P403+P233 | |||||||||
| Hazard Codes | T+,Xn,F | |||||||||
| Risk Statements | 26/27/28-36-20/21/22-11 | |||||||||
| Safety Statements | 22-36/37/39-45-36/37-26-16 | |||||||||
| RIDADR | UN 1544 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QC8050000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 i.v. in mice: 59 mmol/kg (Umans, Inturrisi) | |||||||||
| NFPA 704 |
|
HEROIN Chemical Properties,Uses,Production
Chemical Properties
Light Brown Solid
Uses
Heroin does not occur in opium. It is madefrom poppy extract. Acetylation of morphinewith acetic anhydride or acetyl chlorideproduces heroin. Heroin is not generally usedclinically. Its illicit use in the street has beenrampant.
Uses
Narcotic analgesic prepared from morphine and acetic anhydride. Controlled substance (opium derivative).
Definition
ChEBI: A morphinane alkaloid that is morphine bearing two acetyl substituents on the O-3 and O-6 positions. As with other opioids, heroin is used as both an analgesic and a recreational drug. Frequent and regular administration is associated with tolerance and ph sical dependence, which may develop into addiction. Its use includes treatment for acute pain, such as in severe physical trauma, myocardial infarction, post-surgical pain, and chronic pain, including end-stage cancer and other terminal illnesses.
Biological Functions
Heroin is the diacetyl derivative of morphine. It is not available in the United States for therapeutic use, although its use as a recreational drug is again on the rise. It is either injected or snorted (taken intranasally). It is most often cut, or diluted, with substances such as quinine, which contribute to the flash, or high. Injection of the drug leads to the eventual collapse of the vessels into which it is injected, leading to the appearance of track marks under the skin. Heroin passes rapidly into the brain and thus has a rapid onset of action. It is then metabolized to morphine. The rapid onset contributes to the abuse liability of the drug.Heroin use in pregnant women can lead to low-birth-weight babies, babies born addicted to heroin, immunosuppression, and an increased incidence of infections in both the mother and newborn; an increased incidence of AIDS also occurs.
General Description
Heroin, was first commercially synthesized in 1898 byBayer company in Germany as an alternate analgesic tomorphine. Heroin is the 3,6 diacetylated form of morphine. The laboratory researchers, which also used theacetylation process to convert salicylic acid into acetylsalicylic acid (aspirin), believed that heroin would be an effectiveanalgesic with no addictive properties. This was unfortunatelynot the case. They named the product “heroin”because it made the test subjects, including some of thechemists, feel “heroic.” With both OH groups protected asan ester, heroin can pass through the blood-brain barrierquicker than morphine and lead to the euphoric “rush” thatbecomes so addictive to addicts, especially after IV injection.Once heroin is in the brain, it is quickly metabolizedto 3-acetylmorphine, which has low to zero activity at theμ-receptor and 6-acetylmorphine, which is 2 to 3 timesmore potent at the μ-receptor than morphine.
Heroin is not available as a prescription product in theUnited States, although it is available in some countries totreat pain associated with cancer and myocardial infarctions.It remains one of the most widely used narcotics for illicitpurposes and places major economic burdens on society.
Health Hazard
Heroin is a strongly habit-forming drug. Itis more potent than its parent compound,morphine, and much more potent thanopium. The toxic effects are similar tothose of morphine. An overdose can causerespiratory failure. It is a depressant anda stimulant of the central nervous system,showing a wide range of effects, rangingfrom drowsiness to distorted perceptionsand hallucinations. Toxicological problemsfrom an overdose of heroin may also leadto coma and pinpoint pupil. The toxicityof heroin may be enhanced when it iscoadministered with cocaine. Administrationof a narcotic antagonist such as naloxone ornaltrexone may be performed for therapy.A subcutaneous lethal dose in rabbits maybe 180 mg/kg. Heroin may produce harmfulreproductive effects in animals and humans.It is a controlled substance (opiate) listedunder the U.S. Code of Federal Regulations(Title 21, Part 329.1, 1308.11, 1987).
Pharmacology
Diamorphine is available for parenteral and oral use. It is more lipid soluble
than morphine, affecting distribution and tissue penetration. One advantage
over morphine is in seings where high concentrations are required in
relatively low volumes, such as palliative care. Furthermore, when lipid
solubility is important in regulating site of action (e.g. epidural, intrathecal
use), some practitioners believe that diamorphine has specific advantages
over morphine.
Diamorphine is a prodrug. It is inactive at opioid receptors but is
converted rapidly to the active metabolites 6-monoacetylmorphine (6 MAM),
morphine and M-6-G. Presence of 6-MAM in urine or salvia can be used to
differentiate between morphine and heroin (diamorphine) consumption.
Further metabolism is similar to that of morphine; similar
problems may arise in renal impairment.
HEROIN Preparation Products And Raw materials
Raw materials
Preparation Products
1of2