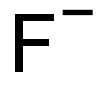FLUORIDE STANDARD
- CAS No.
- 16984-48-8
- Chemical Name:
- FLUORIDE STANDARD
- Synonyms
- FLUORIDE;FluorionF-;Perfluoride;FLUORIDEGEL;Chebi:17051;FLUORIDE ION;Fluoride(1-);Fluorine, ion;fluoride fume;Fluorine anion
- CBNumber:
- CB8310763
- Molecular Formula:
- F-
Lewis structure

- Molecular Weight:
- 19
- MDL Number:
- MFCD00144315
- MOL File:
- 16984-48-8.mol
- MSDS File:
- SDS
| Melting point | 135℃ |
|---|---|
| Density | 1.000 g/cm3 (20 °C) |
| refractive index | 1.3358 (589.3 nm 20℃) |
| storage temp. | 2-8°C |
| form | Liquid |
| PH | 6 (H2O, 20°C) |
| Water Solubility | Immiscible with water. |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| EPA Primary Drinking Water Standard | MCL:4,MCLG:4 |
| CAS DataBase Reference | 16984-48-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | fluoride |
| FDA UNII | Q80VPU408O |
| ATC code | A12CD51 |
| IARC | 3 (Vol. 27, Sup 7) 1987 |
| EPA Substance Registry System | Fluoride (16984-48-8) |
SAFETY
Risk and Safety Statements
FLUORIDE STANDARD price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1.19814 | Fluoride standard solution traceable to SRM from NIST NaF in H?O 1000 mg/l F Certipur? | 16984-48-8 | 500mL | $68.5 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | CHM0297082 | FLUORIDE 95.00% | 16984-48-8 | 5MG | $504 | 2021-12-16 | Buy |
FLUORIDE STANDARD Chemical Properties,Uses,Production
Description
Of the general formula MxFy, appearance,odor and properties vary with specific compounds.
Chemical Properties
Of the general formula FyMx or MxFy, appear ance, odor and properties vary with specific compounds.
Uses
Fluoride, Ion chromatography standard solution, Specpure|r, F|- 1000μg/ml is used as a standard solution in analytical chemistry and in ion chromatography. It is used for calibration of ion chromatography and other analytical techniques.
Definition
Any inorganic salt of hydrofluoric acid in which fluorine has a valence of ?1. Fluorides are major environmental pollutants released into the atmosphere from aluminum reduction, steel manufacturing, and coal-fired power plants. Fluoride pollution is assoc
Hazard
Highly toxic; poison; mutagen; can cause convulsions, changes in the respiratory system, liver and kidneys.
Health Hazard
Small amounts of fluoride appear to be an essential nutrient. People in the United States ingest about 2 mg/day in water and food. A concentration of about 1 mg/L in drinking water effectively reduces dental caries without harmful effects on health. Dental fluorosis can result from exposure to concentrations above 2 mg/L in children up to about 8 years of age. In its mild form, fluorosis is characterized by white opaque mottled areas on tooth surfaces. Severe fluorosis causes brown to black stains and pitting. Although the matter is controversial, the EPA has determined that dental fluorosis is a cosmetic and not a toxic or an adverse health effect. Water hardness limits fluoride toxicity to humans and fish. The severity of fluorosis decreases in harder drinking water. Crippling skeletal fluorosis in adults requires the consumption of about 20 mg or more of fluoride per day over a 20-year period. No cases of crippling skeletal fluorosis have been observed in the United States from the longterm consumption of 2 L/day of water containing 4 mg/L of fluoride. The EPA has concluded that 0.12 mg/kg/day of fluoride is protective of crippling skeletal fluorosis. Fluoride therapy, where 20 mg/day is ingested for medical purposes, is sometimes used to strengthen bone, particularly spinal bones.
Potential Exposure
Fluorides are used as an electrolyte in aluminum manufacture; a flux in smelting nickel, copper, gold, and silver; as a catalyst for organic reactions, a wood preservative; fluoridation agent for drinking water; a bleaching agent for cane seats; in pesticides, rodenticides, and as a fermentation inhibitor. They are utilized in the manufacture of steel, iron, glass, ceramics, pottery, enam els, in the coagulation of latex; in coatings for welding rods; and in cleaning graphite, metals, windows, and glass ware. Exposure to fluorides may also occur during prepara tion of fertilizer from phosphate rock by addition of sulfuric acid. Air pollution by fluoride dusts and gases has done substantial damage to vegetation and to animals in the vicinity of industrial fluoride sources. However, the contri bution of ambient air to human fluoride intake is only a few hundredths of a milligram per day, an amount that is insignificant in comparison with other sources of fluoride. Operations that introduce fluoride dusts and gases into the atmosphere include: Grinding, drying, and calcining of fluoride-containing minerals; acidulation of the minerals; smelting; electrochemical reduction of metals with fluoride fluxes or melts, as in the aluminum and steel industry; kiln firing of brick and other clay products and the combustion of coal.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith large amounts of soap and water. Seek medical attention immediately. If this chemical has been inhaled, removefrom exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantitiesof water and induce vomiting. Do not make an unconsciousperson vomit. Medical observation is recommended for24-48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corticosteroid spray.
storage
Storage color will depend on compound. Prior toworking with fluorides you should be trained on its properhandling and storage. Fluorides must be stored to avoidcontact with strong acids (i.e., hydrochloric, sulfuric, andnitric) since violent reactions can occur. Fluorides formexplosive gases on contact with nitric acid. Store in tightlyclosed containers in a cool, well-ventilated area away fromwater.
Incompatibilities
Fluorides form explosive gases on con tact with strong acids or acid fumes.
Waste Disposal
Reaction of aqueous waste with an excess of lime, followed by lagooning; and either recovery or land disposal of the separated calcium fluoride.
FLUORIDE STANDARD Preparation Products And Raw materials
FLUORIDE STANDARD Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| BeiJing Hwrk Chemicals Limted | 0757-86329057 18934348241 | sales4.gd@hwrkchemical.com | China | 14067 | 55 |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 400-6206333 18521732826; | market@aladdin-e.com | China | 25015 | 65 |
| The future of Shanghai Industrial Co., Ltd. | 021-61552785 | sales@shshiji.com | China | 9552 | 55 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 | cdhxsj@163.com | China | 13358 | 58 |
| Shanghai Macklin Biochemical Co.,Ltd. | 15221275939 15221275939 | shenlinxing@macklin.cn | China | 15878 | 55 |
| Sigma-Aldrich | 021-61415566 800-8193336 | orderCN@merckgroup.com | China | 51471 | 80 |
| Codow Chemical Co.,Ltd. | 18620099427 18620099427 | amy@howeipharm.com | China | 1751 | 55 |
| Supplier | Advantage |
|---|---|
| Shanghai Acmec Biochemical Technology Co., Ltd. | 58 |
| Aladdin Scientific | 58 |
| Mainchem Co., Ltd. | 55 |
| BeiJing Hwrk Chemicals Limted | 55 |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 65 |
| The future of Shanghai Industrial Co., Ltd. | 55 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 58 |
| Shanghai Macklin Biochemical Co.,Ltd. | 55 |
| Sigma-Aldrich | 80 |
| Codow Chemical Co.,Ltd. | 55 |
Related articles
- Formation of Fluoride Ions and Their Charges
- Fluoride ions carry a single negative charge, which makes them useful in a variety of chemical applications such as complex sy....
- Feb 5,2024
16984-48-8(FLUORIDE STANDARD)Related Search:
1of4