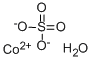Cobalt sulfate
- CAS No.
- 10124-43-3
- Chemical Name:
- Cobalt sulfate
- Synonyms
- Cobalt Sulphate;CoSO4;Cobalt(ii) sulphate;C026305;cobaltbrown;Cobaltsulfat;Cobalt sulfate;COBATOUSSULPHATE;Kobalt(II)-sulfat;cobalt(2+)sulfate
- CBNumber:
- CB8497482
- Molecular Formula:
- CoO4S
- Molecular Weight:
- 155
- MDL Number:
- MFCD00010943
- MOL File:
- 10124-43-3.mol
| Melting point | decomposes at 1140℃ [JAN85] |
|---|---|
| Density | d425 3.71 |
| vapor pressure | 0Pa at 20℃ |
| solubility | H2O: soluble |
| form | red orthorhombic crystals |
| color | red orthorhombic crystals, crystalline |
| Water Solubility | dissolves slowly in boiling H2O [MER06]; g/100g solution H2O: 19.7 ± 0.1 (0°C), 27.2 ± 0.1 (25°C), 27.8 (100°C); solid phase, CoSO4 · 7H2O (0°C, 25°C), CoSO4 ·H2O (100°C) [KRU93] |
| Merck | 13,2473 |
| InChI | InChI=1S/Co.H2O4S.H2O/c;1-5(2,3)4;/h;(H2,1,2,3,4);1H2/q+2;;/p-2 |
| InChIKey | BGORGFZEVHFAQU-UHFFFAOYSA-L |
| SMILES | S([O-])([O-])(=O)=O.O.[Co+2] |
| LogP | -1.031 (est) |
| Substances Added to Food (formerly EAFUS) | COBALT SULFATE--PROHIBITED WITH EXCEPTIONS |
| FDA 21 CFR | 582.80 |
| CAS DataBase Reference | 10124-43-3(CAS DataBase Reference) |
| EWG's Food Scores | 4 |
| FDA UNII | H7965X29HX |
| Proposition 65 List | Cobalt Sulfate |
| NIST Chemistry Reference | Cobalt sulfate(10124-43-3) |
| EPA Substance Registry System | Cobalt(II) sulfate (10124-43-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H290-H317-H319-H334-H341-H350i-H360F-H410 |
| Precautionary statements | P201-P261-P280-P284-P304+P340-P308+P313 |
| Hazard Codes | T,N |
| Risk Statements | 49-42/43-51/53-50/53-22-68-60 |
| Safety Statements | 53-23-36/37-45-61-60-22 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 2 |
Cobalt sulfate price More Price(1)
Cobalt sulfate Chemical Properties,Uses,Production
Physical Properties
The anhydrous salt of cobalt(II) sulfate is a red orthogonal crystal; density 3.71g/cm3; melts above 700°C; the monohydrate is red orthogonal crystal having a density of 3.08 g/cm3; the heptahydrate is a pink salt, monoclinic prismatic crystals, density 2.03 g/cm3; heptahydrate dehydrates to hexahydrate at 41°C and converts to monohydrate at 74°C; the anhydrous salt and heptahydrates are soluble in water; monohydrate slowly dissolves in boiling water.
Uses
Cobalt(II) sulfate is used in storage batteries and electroplating baths for cobalt. It also is used as a dryer for lithographic inks; in pigments for decorating porcelains; in ceramics, glazes and enamels to protect from discoloring; and as a additive to soils.
Preparation
Cobalt(II) sulfate is prepared by dissolving cobalt(II) oxide, hydroxide or carbonate in dilute sulfuric acid, followed by crystallization:
CoO + H2SO4 → CoSO4 + H2O
Co(OH)2 + H2SO4 → CoSO4 + 2H2O
CoCO3 + H2SO4 → CoSO4 + CO2 + H2O
Crystallization yields the commercial product, pink heptahydrate. Further oxidation of this salt in dilute H2SO4 with ozone or fluorine produces hydrated cobalt(III) sulfate, Co2(SO4)3•18H2O. This blue octadecahydrate, Co2(SO4)3•18H2O also is obtained by electrolytic oxidation of cobalt(II) chloride or any cobalt(II) salt solution in 8M sulfuric acid.
Description
The blue, crystalline hydrate Co2(SO4)3.18H2O is prepared by the oxidation of cobalt(II) sulphate in 8N sulphuric acid either electrolytically or chemically with ozone or fluorine. It is stable in the dry state, but is decomposed by water with evolution of oxygen; it is fairly stable in solution in dilute sulphuric acid. Cobalt(III) alums MCo(SO4)2.12H2O (M = K, Rb, Cs or NH4) can be isolated as blue crystals from the mixed cooler solutions of the two sulphates in dilute sulphuric acid. The potassium alum is diamagnetic, the rubidium salt has a magnetic moment less than 1 B.M. and the ammonium alum has a moment of 2.1 B.M. at 304°K. The hydrated sulphate also has a small positive magnetic susceptibility. The sulphate is believed like the alums to contain the [Co(H2O)6]3+ ion.
Chemical Properties
Red powder or rose pink crystalline solid. Odorless.
Uses
Cobalt(II) Sulphate is used in batteries and fuel cells that display long-term stability.
Uses
Ceramics, pigments, glazes, in plating baths for cobalt, additive to soils, catalyst, paint and ink drier, storage batteries.
Definition
ChEBI: Cobalt(2+) sulfate is a compound of cobalt and sulfate in which the ratio of cobalt (+2 oxidation state) to sulfate is 1:1. It contains a cobalt(2+).
General Description
Odorless rose-pink solid. Sinks and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic salts, such as Cobalt sulfate , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
May not be used in food products (FDA). Possible carcinogen.
Health Hazard
Inhalation causes shortness of breath and coughing; permanent disability may occur. Ingestion causes pain and vomiting. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic cobalt oxide fumes may form in fire.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. Questionable carcinogen. When heated to decomposition it emits toxic fumes of SOx See also COBALT COMPOUNDS.
Potential Exposure
Many be used to catalyze organic reactions.
Carcinogenicity
Cobalt sulfate is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it three times from conductivity water (1.3mL/g) between 100o and 0o depending on which hydrate is required. The heptahydrate crystallises below 44o and is efflorescent with m 97o . Between 44o and 70o the monoclinic hexahydrate CoSO4.6H2O m 41.5o is formed, and above 70o the monohydrate CoSO4.H2O m 71o is obtained. The pale reddish or lavender-coloured anhydrous salt is obtained by heating the hydrate above 250o, boiling with conc H2SO4 or heating with (NH4)2SO4).
Incompatibilities
Aqueous solution reacts with bases, generating some heat. May react as either oxidizing agents or reducing agents
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed.
Cobalt sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| henan kanbei chemical co.,ltd | +undefined-86-1523780-4566 +undefined15237804566 | henankanbeichemical@163.com | China | 511 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Cobalt sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-18 | Cobalt sulfate
10124-43-3
|
US $1.00 / kg | 1kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2023-10-17 | Cobalt sulfate
10124-43-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-10-17 | Cobalt sulfate
10124-43-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |
-

- Cobalt sulfate
10124-43-3
- US $1.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Cobalt sulfate
10124-43-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Cobalt sulfate
10124-43-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd