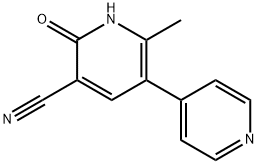
Milrinone
- CAS No.
- 78415-72-2
- Chemical Name:
- Milrinone
- Synonyms
- Primacor;Milirinone;Milrinone USP;YM-018;Minong;Milrila;Corotrop;win47203;Mirinone;Corotrope
- CBNumber:
- CB8695411
- Molecular Formula:
- C12H9N3O
- Molecular Weight:
- 211.22
- MDL Number:
- MFCD00133539
- MOL File:
- 78415-72-2.mol
- MSDS File:
- SDS
| Melting point | >3000C |
|---|---|
| Boiling point | 350.9°C (rough estimate) |
| Density | 1.1922 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: >10 mg/mL |
| form | powder |
| pka | 7.21±0.10(Predicted) |
| color | off-white |
| Water Solubility | Soluble in DMSO. Insoluble in water. |
| Merck | 14,6197 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | FYEJSUDMDUIMKJ-UHFFFAOYSA-N |
| CAS DataBase Reference | 78415-72-2(CAS DataBase Reference) |
| FDA UNII | JU9YAX04C7 |
| ATC code | C01CE02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301 | |||||||||
| Precautionary statements | P301+P330+P331+P310 | |||||||||
| Hazard Codes | T,T+ | |||||||||
| Risk Statements | 23/24/25-26/27/28 | |||||||||
| Safety Statements | 36/37/39-45-22 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DW1762000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29337900 | |||||||||
| NFPA 704 |
|
Milrinone price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2515 | Milrinone Pharmaceutical Secondary Standard; Certified Reference Material | 78415-72-2 | 1G | $334 | 2024-03-01 | Buy |
| Sigma-Aldrich | 475840 | Milrinone | 78415-72-2 | 10mg | $332 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1443908 | Milrinone United States Pharmacopeia (USP) Reference Standard | 78415-72-2 | 500mg | $732 | 2024-03-01 | Buy |
| TCI Chemical | M1663 | Milrinone >98.0%(N) | 78415-72-2 | 100mg | $101 | 2024-03-01 | Buy |
| TCI Chemical | M1663 | Milrinone >98.0%(N) | 78415-72-2 | 1g | $401 | 2024-03-01 | Buy |
Milrinone Chemical Properties,Uses,Production
Positive Inotropic drugs
Milrinone, also known as bipyridine ketone, methyl cyanide topiramate ketone, is a drug for the treatment of heart failure ,it is a homolog of amrinone, having positive inotropic and vasodilatory effects, its mechanism of action is the same as ammonia milrinone , but the strength of its effect is 10 to 30 times of amrinone,without side effects of reducing platelets,it is well tolerated. Oral or intravenous way has a good effect, it can increase cardiac output and cardiac index, reduce left ventricular end diastolic pressure, pulmonary wedge pressure and right atrial pressure, it has no significant effect on blood pressure and heart rate,it takes effect within 0.5 hours after oral administration,and achieves to maximum effect after 1 to 3 hours , the half-life is 2 hours, the effect can be maintained for 4-6 hours, it is mainly metabolically inactivated in the liver , 80% of it is excreted unchanged in the urine. It is clinically used in the treatment of various acute and chronic heart failure,it is effective in digitalis and diuretics invalid refractory heart failure . This product is the same as amrinone, significant short-term effect, long-term efficacy remains to be further confirmed, but the side effects are little.
Compared with amrinone, milrinone has the following characteristics:
1, small doses mainly have positive inotropic effect, vasodilator effect is gradually enhanced while increasing the dose ,its positive inotropic effect accounts for 1/3 of the total effect in the treatment of heart failure, vasodilation effect accounts for2/3 of the total effect.
2,not significantly increasing myocardial oxygen consumption.
3, to improve peripheral circulatory disorders in heart failure, to increase exercise capacity of patients with heart failure and improve their quality of life.
4, for cardiac β receptors in a low-key and reduced β agonistssusceptibility heart failure patients, the drug is still valid.
5, the positive inotropic effect does not produce tachyphylaxis phenomenon.
6, no significant effect on blood pressure and heart rate.
The above information is edited by the Chemicalbook of Tian Ye.
Mechanism
Milrinone is non-digitalis, non-catecholamines cardiac drug , it is representative drug of phosphodiesterase inhibitor,selectively inhibiting phosphodiesterase in myocardial cells, increasing intracellular cyclic adenosine monophosphate, alter intracellular calcium transport, thereby increasing myocardial contractility and expanding peripheral vessel . An increase in myocardial oxygen consumption caused by Milrinone increasing myocardial contractility may be offset by its vasodilator effect. Milrinone is an ideal drug for the treatment of coronary artery stenosis, decompensated heart failure led by myocardial ischemia . Because milrinone hasβ receptor down features, it is an ideal drug for the treatment of decompensated heart failure in patients applying β-blocker . Because milrinone has effect on expanding small arteries and veins, it is more beneficial than dobutamine for the traetment of decompensated heart failure with severe pulmonary hypertension .
Dosage
First dose is slow intravenous injection (50 μg/kg, intravenous injection after dilution, time is not less than 10 min), then with 0.375~0.750 μg/(kg · min), continuous intravenous drip. The maximum daily dose is 1.13 mg/kg. Patients with renal insufficiency reduce the use according to creatinine clearance level, for example, creatinine clearance rate is 20 ml/(min · 1.73 m2), intravenously at a dose of 0.28 μg/(kg · min). 80% of Milrinone is excreted from the kidneys, in general,for renal insufficiency patients, the dose should be halved.
Drug Interactions
Patients already receiving angiotensin-converting enzyme inhibitor therapy using milrinone can further improve hemodynamics, but also increase the side effects caused by the blood vessels dilation. Milrinone combinated with mid-dose dobutamine, can enhance the positive inotropic effect, further reducing left ventricular end-diastolic pressure. When there is low blood pressure, milrinone can theoretically combine a larger dose dopamine.
Precautions
1, showing ventricular arrhythmia, supraventricular arrhythmias; few are headache, ventricular arrhythmias, weakness, low platelet count, etc; overdose may lead to low blood pressure, tachycardia; occasionally bronchospasm, fever .
2, acute myocardial infarction patients are disabled.
3, patients with hypotension, tachycardia, renal insufficiency, atrial fibrillation or flutter, electrolyte imbalance, drug-induced arrhythmias, kidney disease, severe aortic or pulmonary valve disease , pregnant women, lactating women should use with caution.
4,the elimination half-life of this product is significantly prolonged in patients with renal insufficiency , it should be reduced in patients with renal insufficiency, and the infusion rate should be slow . Elderly patients do not need to adjust the dose.
Chemical Properties
Crystallization From Dimethyl formamide monohydrate or ethanol, the melting point> 300 ℃.
Uses
New non-glycosides non-catecholamines cardiac drug , its function is similar to amrinone ,positive inotropic effect is strong and about 20 to 50 times of amrinone, and it has significant effect on expanding smooth muscle,it can reduce the load on the heart, it can also improve kidney and muscle blood supply. Oral and intravenous are valid, no serious adverse reactions. It is used For severe heart failure such as chronic heart failure and congestive heart failure.
production method
Method 1: 4-methylpyridine is dissolved in anhydrous diethyl ether, under protection of nitrogen, a solution of phenyl lithium in diethyl ether is added dropwise with stirring at room temperature, the reaction is continued. Nitrogen is stopped, cool to 0 ℃, ethyl acetate is added dropwise at below 10 ℃. With concentrated hydrochloric acid to Ph = 1~2, the aqueous layer is separated, basify with saturated sodium carbonate to Ph = 9, precipitate oil , extract several times with chloroform, dry, chloroform is distilled off, vacuum distillation, collecting 130-132 ℃/1.47kPa of fractions, namely, 1-(4-pyridyl) acetone.
1-(4-pyridyl) acetone, acetonitrile, and N, N-dimethylformamide dimethyl acetal are refluxed together. The solvent is distilled off under reduced pressure, the residue is dissolved in an appropriate amount of chloroform and an alumina column (Soxhlet)is heated at reflux for extraction, concentrate extract, with carbon tetrachloride-cyclohexane (4: 1) recrystallize to give 1-(4-pyridyl)-2-(dimethylamino) ethenyl methyl ketone.
1-(4-pyridyl)-2-(dimethylamino) ethenyl methyl ketone, cyano acetamide, sodium methoxide and dimethyl formamide are heated under reflux, the solvent is distilled off under reduced pressure, the residue is added acetonitrile, warm to dissolve , cool to 10 ℃; the precipitated solid is filtered, dissolve with amount of water, decolorize with charcoal, and acidify with hydrochloric acid 6mol/L to Ph = 6.5~7, the precipitated solid is recrystallized from dimethylformamide to give pale yellow granular crystals, which are milrinone, mp> 300 ℃.
Method 2: use 4-pyridyl acetone as a raw material, after condensation with triethyl orthoformate, it reacts with 2-cyanoacetamide, to obtain milrinone.
Method 3: pyridyl acetone reacts with 2,2-cyano-vinyl ethyl ether reaction to generate milrinone.
Description
Milrinone, an inhibitor of phosphodiesterase selective for fraction III PDE, exerts a positive inotropic effect on the heart as well as a peripheral vasodilatory effect. Milrinone given intravenously produces significant improvements on cardiac output, pulmonary capillary wedge pressure and vascular resistance without significant effects on the heart rate.
Description
Milrinone is an inhibitor of type 3 phosphodiesterases (PDEs), inhibiting recombinant PDE3A and PDE3B with IC50 values of 0.45 and 1 μM, respectively. It is selective for PDE3 over PDE1, PDE2, PDE4, PDE5, and PDE7 (IC50s = 263, >300, 17.5, 49.1, and 58.3 μM, respectively). Milrinone (0.1-1 mg/kg) has positive inotropic effects, increasing cardiac contractile force in anesthetized dogs with a concomitant increase in heart rate but not blood pressure. It also increases contractile force in models of propranolol- and verapamil-induced heart failure in anesthetized dogs when administered at an initial dose of 30 μg/kg followed by a continuous 3 μg/kg per minute infusion. Milrinone has vasodilatory effects as well, decreasing mean aortic pressure and increasing venous compliance in anesthetized dogs when administered at an initial dose of 10 μg/kg followed by a continuous 1.7-2.4 μg/kg per minute infusion. Formulations containing milrinone have been used in the treatment of heart failure.
Chemical Properties
Crystalline Solid
Originator
Eastman Kodak (Sterling) (USA)
Uses
It is used (particularly intravenously, as the lactate) for the short-term management of severe heart failure. Milrinone inhibits the action of phosphodiesterase-3 preventing degradation of cAMP. The corresponding increase in cAMP levels leads to increased activation of protein kinase A. It is a selective phosphodiesterase inhibitor with vasodilating and positive inotropic activity.
Uses
bronchodilator
Uses
antidepressant, 5HT reuptake inhibitor
Uses
Selective phosphodiesterase inhibitor with vasodilating and positive inotropic activity. Cardiotonic
Definition
ChEBI: Milrinone is a member of the class of bipyridines that is 2-pyridone which is substituted at positions 3, 5, and 6 by cyano, pyrid-4-yl, and methyl groups, respectively. It is used (particularly intravenously, as the lactate) for the short-term management of severe heart failure. It has a role as an EC 3.1.4.17 (3',5'-cyclic-nucleotide phosphodiesterase) inhibitor, a platelet aggregation inhibitor, a vasodilator agent and a cardiotonic drug. It is a pyridone, a nitrile and a member of bipyridines.
Manufacturing Process
A mixture containing 20 g of 1-(4-pyridinyl)-2-propanone and 30 ml ofhexamethylphosphoramide was diluted with 65 ml of dimethylformamidedimethyl acetal and the resulting mixture was refluxed for 30 min. TLCanalysis showed a single spot, thereby indicating completion of the reaction(in another run, the reaction appeared to be complete after 30 min at roomtemperature). The mixture was evaporated under reduced pressure and apressure, thereby resulting in a crystalline residue weighing 24 g. The residuewas purified by continuous chromatographic extraction on alumina (about 150g) using refluxing chloroform as eluant. After 90 min, the extract was heatedin vacuo to remove the chloroform, thereby leaving, as a light yellowcrystalline material, 23.2 g of 1-(4-pyridinyl)-2-(dimethylamino)ethenylmethyl ketone, alternatively named 4-dimethylamino-2-(4-pyridinyl)-3-buten-2-one.
To a mixture containing 23 g of 1-(4-pyridinyl)-2-(dimethylamino)ethenylmethyl ketone and 11 g of α-cyanoacetamide dissolved in 400 ml ofdimethylformamide was added with stirring 14 g of sodium methoxide and theresulting reaction mixture was heated in an oil bath under gentle reflux forone hour. TLC analysis showed no starting material in the reaction mixturewhich was then concentrated in vacuo on a rotary evaporator to a volume ofabout 80 ml. The concentrate was treated with about 160 ml of acetonitrileand the resulting mixture was stirred on a rotary evaporator with warminguntil homogenous and then cooled. The crystalline product was collected,rinsed successively with acetonitrile and ether, and dried overnight at 55°C toyield 28 g of crystalline product, namely, sodium salt of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile, the presence of cyano being confirmed byIR analysis. An 8 g portion of said sodium salt was dissolved in 75 ml of hotwater, the aqueous solution treated with decolorizing charcoal, filtered, thefiltrate again treated with decolorizing charcoal and filtered, and the filtrateacidified with 6 N hydrochloric acid by dropwise addition to a pH of 3. Theacidic mixture was diluted with ethanol and cooled. The crystalline productwas collected, dried, recrystallized from dimethylformamide-water and dried toproduce 3.75 g of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile,m.p. >300°C.
Another method of preparation of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile (Patent US 4,413,127)
A 69.5 g portion of 1-ethoxy-2-(4-pyridinyl)ethenyl methyl ketone wasdissolved in 300 ml of ethanol and to the solution was added 13.2 g ofmalononitrile. The resulting mixture was refluxed for 5 hours, crystals startingto separate after about 30 min of refluxing. The reaction mixture was allowedto cool to room temperature and, the precipitate of fine needles was filtered,washed with ethanol and dried in a vacuum at 90°C to yield 25.4 g of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)-nicotinonitrile, m.p. >300°C.Concentration of the mother liquor provided another 2.1 g of product, m.p.>300°C.
To a aqueous solution of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile was added one molar equivalent of lactic acid to prepare themonolactate of 1,2-dihydro-6-methyl-2-oxo-5-(4-pyridinyl)nicotinonitrile.
brand name
Primacor (Sterling Winthrop);Corotrope.
Therapeutic Function
Cardiotonic
General Description
Milrinone, 1,6-dihydro-2-methyl-6-oxo-3,4'-bipyridine-5-carbonitrile (Primacor), is another dipyridinephosphodiesterase F-III inhibitor that possesses pharmacologicalproperties similar to those of amrinone. The inhibitionof the degradation of cAMP results in an increase in the cardiacmuscle’s force of contraction.
Biological Activity
Potent cAMP phosphodiesterase inhibitor (IC 50 = 56 nM for inhibition of PDE III). Has inotropic and vasodilator effects following oral or intravenous administration in vivo . Also available as part of the Phosphodiesterase Inhibitor Tocriset™ .
Biochem/physiol Actions
Phosphodiesterase type III inhibitor; cAMP-specific, cGMP-inhibitable; potent cardiotonic, positive inotropic vasodilator.
storage
Store at +4°C
References
[1] tang km1, jang ek, haslam rj. photoaffinity labelling of cyclic gmp-inhibited phosphodiesterase (pde iii) in human and rat platelets and rat tissues: effects of phosphodiesterase inhibitors. eur j pharmacol. 1994 jun 15;268(1):105-14.
[2] cheung p1, yang g, boden g. milrinone, a selective phosphodiesterase 3 inhibitor, stimulates lipolysis, endogenous glucose production, and insulin secretion. metabolism. 2003 nov;52(11):1496-500.
Milrinone Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Beijing Yibai Biotechnology Co., Ltd | 0086-182-6772-3597 | sales04@yibaibiotech.com | CHINA | 419 | 58 |
View Lastest Price from Milrinone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Milrinone
78415-72-2
|
US $670.00-530.00 / g | 1g | 99% | 30kg | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-22 | Milrinone
78415-72-2
|
US $0.00 / Kg/Bag | 2Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-22 | Milrinone
78415-72-2
|
US $0.00 / Kg/Bag | 2Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. |
78415-72-2(Milrinone)Related Search:
1of4