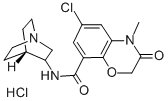
Azasetron hydrochloride
- CAS No.
- 123040-16-4
- Chemical Name:
- Azasetron hydrochloride
- Synonyms
- Y-25130;Serotone;AZASETRON;Y-25130HCl;AZASETRON HCL;PubChem CID 2264;Y-25130 HYDROCHLORIDE;Azasetron hydrochloride;Azasetron HCl (Y-25130);INTERMEDIATE OF AZASETRON HCL
- CBNumber:
- CB8706748
- Molecular Formula:
- C17H21Cl2N3O3
- Molecular Weight:
- 386.27
- MDL Number:
- MFCD00209913
- MOL File:
- 123040-16-4.mol
- MSDS File:
- SDS
| Melting point | 281° (dec); mp 305° (dec) (Kawakita) |
|---|---|
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | White solid. |
| color | White |
| CAS DataBase Reference | 123040-16-4(CAS DataBase Reference) |
| FDA UNII | 2BSS7XL60S |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 |
| Toxicity | LD50 in male, female rats (mg/kg): 135, 132 i.v. (Takagi) |
Azasetron hydrochloride price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML2491 | Azasetron hydrochloride ≥98% (HPLC) | 123040-16-4 | 5mg | $102 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML2491 | Azasetron hydrochloride ≥98% (HPLC) | 123040-16-4 | 25mg | $411 | 2024-03-01 | Buy |
| Cayman Chemical | 28383 | Azasetron (hydrochloride) | 123040-16-4 | 10mg | $97 | 2024-03-01 | Buy |
| Cayman Chemical | 28383 | Azasetron (hydrochloride) | 123040-16-4 | 100mg | $625 | 2024-03-01 | Buy |
| Cayman Chemical | 28383 | Azasetron (hydrochloride) | 123040-16-4 | 25mg | $218 | 2024-03-01 | Buy |
Azasetron hydrochloride Chemical Properties,Uses,Production
Description
Nazasetron (azasetron) hydrochloride is the fourth in the class of highly potent 5- HT3 receptor antagonists to reach the market for prophylaxis of severe nausea and vomiting induced by cytotoxic chemotherapy and by total body X-radiation. In a study with 146 cancer patients suffering from emesis induced by cisplatin, nazasetron was reported to be markedly effective in 72% of subjects. Nazasetron is devoid of dopamine D2 receptor blocking activity and therefore is free of the extrapyrimidal side effects associated with other commonly used antiemetics such as metoclopramide and domperidone. Nazasetron has been reported to be potentially useful for the treatment of gastrointestinal motility dysfunction, such as gastric stasis, vomiting and irritable bowel syndrome.
Chemical Properties
White to Off-White Solid
Originator
Yoshitomi (Japan)
Uses
Azasetron HCl is a selective 5-HT3 receptor antagonist with IC50 of 0.33 nM used in the management of nausea and vomiting induced by cancer chemotherapy.
Uses
A 5-HT3 receptor antagonist. Used as an antiemetic.
Definition
ChEBI: Azasetron hydrochloride is a benzoxazine.
Manufacturing Process
To a solution of 2-(2-carboxy-4-chlorophenoxy)acetic acid in concentrated
sulfuric acid is added dropwise a mixed liquid of fuming nitric acid and
concentrated sulfuric acid under stirring with keeping at a temperature below
10°C. After the addition, the reaction mixture is stirred and poured into 10 L
of ice-cold water. The precipitated crystals are collected by filtration, washed
with 2 L of water four times and them dried to give 2-(2-carboxy-4-chloro-6-
nitrophenoxy)acetic acid.
To a solution of ferrous sulfate heptahydrate in of hot water is added a
solution of 2-(2-carboxy-4-chloro-6-nitrophenoxy)acetic acid and aqueous
concentrated ammonia solution in water under stirring. After stirring, to the
reaction mixture is twice added aqueous concentrated ammonia solution.
While the reaction mixture becomes exothermic, stirring is continued. The
resultant mixture is filtered through a celite layer under reduced pressure and
washed with hot water twice. The filtrate is cooled and made acid with
concentrated hydrochloric acid. The precipitated crystals are washed with
water and dried to give 6-chloro-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-
carboxylic acid.
A mixture of 6-chloro-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic
acid, methanol and concentrated sulfuric acid is refluxed under heating with
stirring, and then cooled. The precipitated crystals are collected by filtration,
washed with methanol and dried to give methyl 6-chloro-3,4-dihydro-3-oxo-
2H-1,4-benzoxazine-8-carboxylate, melting point 239°-241°C.
To a solution of methyl 6-chloro-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylate in dimethylformamide is added potassium t-butoxide and solution
stirred at room temperature. To the resultant solution is added dropwise a
solution of methyl iodide in dimethylformainide under stirring. After the
reaction solution is stirred, water is added thereto. The insoluble substance is
collected by filtration, washed with water and dried to give methyl 6-chloro-
3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carboxylate.
A mixture of methyl 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-
benzoxazine-8-carboxylate, ethanol and 4% aqueous potassium hydroxide
solution is refluxed with heating. The resultant solution is cooled and water is
added thereto followed by filtration. The filtrate is made acid with
concentrated hydrochloric acid. The precipitated crystals are collected by
filtration, washed with water and dried, and then recrystallized from ethanol
to give 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-carboxylic
acid, melting point 241°-243°C.
A solution of 6-chloro-3,4-dihydro-4-methyl-3-oxo-2H-1,4-benzoxazine-8-
carboxylic acid in tetrahydrofuran and dimethylformamide is cooled to below
0°C and triethylamine is added under stirring thereto. Further, ethyl
chlorocarbonate is added and the mixture is stirred at room temperature. To
the resultant mixture is added 3-amino-8-azabicyclo[3.2.1]octane and the
mixture stirred. After completion of the reaction, aqueous sodium hydrogen
carbonate and ethyl acetate are added. The organic layer is separated,
washed with water and dried over magnesium sulfate. The solvent is distilled
off to give 6-chloro-3,4-dihydro-4-methyl-N-(8-azabicyclo[3.2.1]oct-3-yl)-3-
oxo-2H-1,4-benzoxazine-8-carboxamide.
In practice it is usually used as hydrochloride.
brand name
Serotone
Therapeutic Function
Antiemetic
Biological Activity
A selective and potent 5-HT 3 antagonist (K i = 2.9 nM).
storage
Desiccate at -20°C
Azasetron hydrochloride Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | 1056@dideu.com | China | 3683 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
View Lastest Price from Azasetron hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-22 | Azasetron hydrochloride
123040-16-4
|
US $10.00 / kg | 1kg | 99% | 1000tons | Henan Bao Enluo International TradeCo.,LTD | |
 |
2021-11-04 | Azasetron hydrochloride
123040-16-4
|
US $0.00 / Kg/Bag | 1KG | 98%min | 100kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-08-05 | Azasetron hydrochloride USP/EP/BP
123040-16-4
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited |
-

- Azasetron hydrochloride
123040-16-4
- US $10.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD
-

- Azasetron hydrochloride
123040-16-4
- US $0.00 / Kg/Bag
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Azasetron hydrochloride USP/EP/BP
123040-16-4
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited