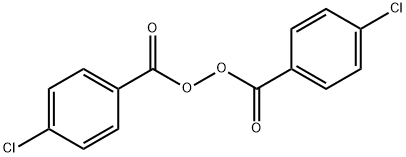
p-Chlorobenzoyl peroxide Bis(p-ehlorobenzoyl)peroxide
- CAS No.
- 94-17-7
- Chemical Name:
- p-Chlorobenzoyl peroxide Bis(p-ehlorobenzoyl)peroxide
- Synonyms
- 4-chlorobenzoyl peroxide;p-Chlorobenzoyl peroxide.;Di-(p-chlorbenzoyl)-peroxid;di-4-chlorobenzoyl peroxide;Di(p-chlorobenzoyl) peroxide;bis(p-chlorobenzoyl)-peroxid;bis(4-chlorobenzoyl) peroxide;bis(p-chlorobenzoyl) peroxide;4-chlorobenzoyl peroxide [qr];Peroxide, bis(4-chlorobenzoyl)
- CBNumber:
- CB8851969
- Molecular Formula:
- C14H8Cl2O4
- Molecular Weight:
- 311.12
- MDL Number:
- MFCD00072660
- MOL File:
- 94-17-7.mol
| Melting point | 137-138 °C |
|---|---|
| Boiling point | 427.4±55.0 °C(Predicted) |
| Density | 1.4614 (estimate) |
| color | A white, granular material |
| Indirect Additives used in Food Contact Substances | CHLOROBENZOYL PEROXIDE, P- |
| FDA UNII | 78776C973U |
| EPA Substance Registry System | Peroxide, bis(4-chlorobenzoyl) (94-17-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS01,GHS07,GHS02 |
|---|---|
| Signal word | Danger |
| Hazard statements | H241-H335-H315-H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362-P210-P220-P234-P280-P370+P378-P370+P380+P375-P403+P235-P411-P420-P501 |
| Toxicity | LDLo ipr-mus: 500 mg/kg CBCCT* 4,110,52 |
p-Chlorobenzoyl peroxide Bis(p-ehlorobenzoyl)peroxide Chemical Properties,Uses,Production
Chemical Properties
White, odorless powder. Decomposes violently on heating or contamination. Insoluble in water; soluble in organic solvents.
Uses
Curing agent for silicone rubbers.
Definition
A mixture of 50% bis(p-chlorobenzoyl)peroxide in silicone fluid.
General Description
Odorless white solid or paste. Sinks in water.
Reactivity Profile
In pure crystalline form there is danger of explosion upon heating, shock, or friction. Peroxides are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion.
Hazard
Dangerous fire and explosion risk, explodes when heated to 38C, strong oxidizer, will ignite on contact with organic materials. Store in dark, cool locality. Toxic.
Health Hazard
Irritates eyes and (on prolonged contact) skin. Ingestion causes irritation of mouth and stomach.
Safety Profile
Moderately toxic byintraperitoneal route. Probably an irritant to skin andmucous membranes. Dangerous fire hazard; a powerfuloxidizer. Store in a cool place away from fire hazards,sparks, open flames, and out of the direct rays of the sun.Dangerous e
Carcinogenicity
The only other data available for this organic peroxide show that it had no effects on tumor cells when injected intraperitoneally at 125mg/kg in the chest wall of mice .
p-Chlorobenzoyl peroxide Bis(p-ehlorobenzoyl)peroxide Preparation Products And Raw materials
Raw materials
Preparation Products
p-Chlorobenzoyl peroxide Bis(p-ehlorobenzoyl)peroxide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Supplier | Advantage |
|---|---|
| Hubei xin bonus chemical co. LTD | 58 |
| Mainchem Co., Ltd. | 55 |