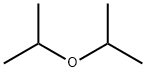
Diisopropyl ether
- CAS No.
- 108-20-3
- Chemical Name:
- Diisopropyl ether
- Synonyms
- DIISOPROPYL ETHER;DIPE;2-ISOPROPOXYPROPANE;2,2'-Oxybispropane;2-propan-2-yloxypropane;ISOPROPYL ETHER, 1000MG, NEAT;(iso-C3H7)2O;Isoprpyl ether;Isopropyl ethe;Diisopropyleth
- CBNumber:
- CB8852739
- Molecular Formula:
- C6H14O
- Molecular Weight:
- 102.17
- MDL Number:
- MFCD00008880
- MOL File:
- 108-20-3.mol
- MSDS File:
- SDS
| Melting point | -85.5 °C |
|---|---|
| Boiling point | 68-69 °C(lit.) |
| Density | 0.725 g/mL at 25 °C(lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 120 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | −29 °F |
| storage temp. | Store below +30°C. |
| solubility | 3.11g/l |
| form | Liquid |
| color | APHA: ≤25 |
| Relative polarity | 2.2 |
| Odor | Sweet, slightly sharp; characteristic pungent; ethereal; like amphor and ethyl ether. |
| explosive limit | 1-21%, 100°F |
| Water Solubility | 9 g/L (20 ºC) |
| Merck | 14,5212 |
| BRN | 1731256 |
| Henry's Law Constant | 2.57 at 25 °C (headspace-GC, Arp and Schmidt, 2004) |
| Exposure limits | TLV-TWA 1045 mg/m3 (250 ppm) (ACGIH), 2090 mg/m3 (500 ppm) (OSHA); STEL 1300 mg/m3 (310 ppm) (ACGIH). |
| Dielectric constant | 3.9(25℃) |
| Stability | Extremely flammable. This material is a serious fire and explosion risk. Vapour may travel considerable distances to an ignition source, which need not be an open flame, but may be a hot plate, steam pipe, etc. Vapour may be ignited by the static electricty which can build up when isopropyl ether is being poured from one vessel |
| LogP | 2.4 at 20℃ |
| CAS DataBase Reference | 108-20-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | DO7Y998826 |
| NIST Chemistry Reference | Diisopropyl ether(108-20-3) |
| EPA Substance Registry System | Isopropyl ether (108-20-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H336 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P243 | |||||||||
| Hazard Codes | F | |||||||||
| Risk Statements | 11-19-66-67-52/53 | |||||||||
| Safety Statements | 9-16-29-33-61 | |||||||||
| RIDADR | UN 1159 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TZ5425000 | |||||||||
| F | 10 | |||||||||
| Autoignition Temperature | 827 °F | |||||||||
| HS Code | 2909 19 90 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 in 14 day old, young adult, adult rats (ml/kg): 6.4, 16.5, 16.0 orally (Kimura) | |||||||||
| IDLA | 1,400 ppm [10% LEL] | |||||||||
| NFPA 704 |
|
Diisopropyl ether price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 95251 | Diisopropyl ether analytical standard | 108-20-3 | 1ML | $42.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 95251 | Diisopropyl ether analytical standard | 108-20-3 | 5ML | $171 | 2024-03-01 | Buy |
| Sigma-Aldrich | 95251 | Diisopropyl ether analytical standard | 108-20-3 | 10ML | $304 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00866 | Diisopropyl ether (stabilized with 2,6-di-tert-butyl-4-methylphenol (BHT)) for synthesis | 108-20-3 | 1L | $50.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00866 | Diisopropyl ether (stabilized with 2,6-di-tert-butyl-4-methylphenol (BHT)) for synthesis | 108-20-3 | 2.5L | $113 | 2024-03-01 | Buy |
Diisopropyl ether Chemical Properties,Uses,Production
Description
Diisopropyl ether [108-20-3], also known as 2-isopropoxyisopropane or isopropyl ether, is a clear, colorless, mobile, highly flammable liquid with a characteristic ethereal odor. Its solubility in water is low, but it is soluble in organic solvents and readily forms explosive peroxides. It has a higher boiling point, a lower vapor pressure, and a lower water solubility than diethyl ether, which means that diisopropyl ether can be recovered without significant loss after use as a solvent or as an extractant.
Chemical Properties
Diisopropyl ether(Isopropyl ether) is highly flammable, with a wide flammable range of 1.4%–21% in air. Boiling point is 156°F (68°C), flash point is 18°F (27°C), and ignition temperature is 830°F (443°C). Vapor density is 3.5, which is heavier than air. In addition to flammability, isopropyl ether is toxic by inhalation and a strong irritant, with a TLV of 250 ppm in air. Diisopropyl ether is an excellent solvent for natural oils, mineral oils, and waxes. It is used as an extracting agent and reaction medium in chemical and pharmaceutical syntheses.
Physical properties
Clear, colorless, volatile, flammable liquid with a penetrating, sweet, ether-like odor. Experimentally determined detection and recognition odor threshold concentrations were 70 μg/m3 (17 ppbv) and 220 μg/m3 (53 ppbv), respectively (Hellman and Small, 1974).
Uses
Isopropyl ether is used as a solvent for oils,waxes, resins, and dyes; and as a varnishremover.
Preparation
Diisopropyl ether can be prepared by liquid-phase dehydration of 2-propanol at 130 – 190°C and 1.96– 7.85 MPa over acidic catalysts containing aluminum.
General Description
Diisopropyl ether appears as a clear colorless liquid with an ethereal odor. Flash point -18°F. Less dense than water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Slightly soluble in water. Form explosive peroxide in storage. A flask of Isopropyl ether was heated on a steam bath with gentle shaking when an explosion occurred. In a second instance, an explosion occurred after practically all the ether had been distilled, [MCA Guide for Safety(1972)].
Reactivity Profile
Ethers, such as Isopropyl ether, can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert. Mixing Isopropyl ether in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid, [NFPA 1991].
Hazard
Flammable, dangerous fire risk, explosive limits in air 1.4–21%. Toxic by inhalation, strong irritant.
Health Hazard
Isopropyl ether is a narcotic and an irritant tothe skin and mucous membranes. It is moretoxic than ethyl ether and the toxic symptomsare similar to the latter compound. Inhalationof its vapors can produce anesthetic effects.Exposure to high concentrations can causeintoxication, respiratory arrest, and death.Exposure to 3.14% and 2.9% of isopropylether in air (by volume) produced the symp toms of somnolence, change in motor activ ity, and muscle contractions in mice andrabbits, respectively, causing the death of50% of test animals. Exposure to 7–10% byvolume concentration in air can be fatal tohumans
Acute oral toxicity of isopropyl ether islow. The liquid is irritating to the mucousmembranes. Skin contact may cause mildirritation, and repeated exposure may causedermatitis. The irritation effect of isopropylether on eyes is mild. In humans, exposureto 800 ppm in air for a few minutes causedirritation of the eyes and nose. There is noreport of its carcinogenic action in animalsor humans.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. Containers may explode when heated.
Flammability and Explosibility
Highly flammable
Industrial uses
Isopropyl ether is a solvent of minor importance since its boiling point is intermediate between the two widely used solvents diethyl ether and acetone. Hazardous peroxides are formed more readily in isopropyl ether than in other dialkyl ethers.
Potential Exposure
ReproductiveEffector; Human Data; Primary Irritant. This material isused as a solvent, additive for gasoline, and a chemicalintermediate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Source
Some unleaded gasoline may contain isopropyl ether and other oxygenates as an octane booster and to prevent engine knock.
Environmental Fate
Biological. Bridié et al. (1979) reported BOD and COD values of 0.19 and 1.75 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C and stirred for a period of 5 d. The ThOD for isopropyl
ether is 2.82 g/g.
Chemical/Physical. May form explosive peroxides on standing with air (NIOSH, 1997).
At an influent concentration of 1,018 mg/L, treatment with GAC resulted in an effluent
concentration of 203 mg/L. The adsorbability of the GAC used was 162 mg/g carbon (Guisti et al.,
1974).
Isopropyl will not hydrolyze in water except at elevated temperatures and low pH.
storage
Isopropyl ether is stored in a flammable-liquids storage room, isolated from combustible and oxidizing materials. It shouldalso be protected from direct sunlight, staticelectricity, and lightning. It is stabilized with0.01% hydroquinone or p-benzylaminphenol.The ether is shipped in amber glass bottles,steel cans, and drums.
Shipping
This compound requires a shipping label of“FLAMMABLE LIQUID.” It falls in Hazard Class 3 andPacking Group II.
Purification Methods
Common impurities are water and peroxides [detected by the liberation of iodine from weakly acid (HCl) solutions of 2% KI]. Peroxides can be removed by shaking with aqueous Na2SO3 or with acidified ferrous sulfate (0.6g FeSO4 and 6mL conc H2SO4 in 110mL of water, using 5-10g of solution per L of ether), or aqueous NaBH4 solution. The ether is then washed with water, dried with CaCl2 and distilled. Alternatively, refluxing with LiAlH4 or CaH2, or drying with CaSO4, then passage through an activated alumina column, can be used to remove water and peroxides. Other dehydrating agents used with isopropyl ether include P2O5, sodium amalgam and sodium wire. (The ether is often stored in brown bottles, or in the dark, with sodium wire.) Bonner and Goishi (J Am Chem Soc 83 85 1961) treated isopropyl ether with dilute sodium dichromate/sulfuric acid solution, followed by repeated shaking with a 1:1 mixture of 6M NaOH and saturated KMnO4. The ether is washed several times with water, dilute aqueous HCl, and water, with a final washing with, and storage over, ferrous ammonium sulfate acidified with H2SO4. Blaustein and Gryder (J Am Chem Soc 79 540 1957), after washing with alkaline KMnO4, then water, treated the ether with ceric nitrate in nitric acid, and again washed it with water. Hydroquinone is added before drying with CaCl2 and MgSO4, and refluxing with sodium amalgam (108g Hg/100g Na) for 2hours under nitrogen. The distillate (nitrogen atmosphere) is made 2 x 10-5M in hydroquinone to inhibit formation of peroxides (which is negligible if the ether is stored in the dark). Catechol (pyrocatechol) and resorcinol are alternative inhibitors. [Beilstein 1 IV 1471.]
Incompatibilities
Forms explosive mixture with air. Aircontact forms explosive peroxides that may explode withheat or shock. Contact with strong oxidizers or strong acidsmay cause a fire and explosion hazard. Attacks some plastics, rubber, and coatings.
Waste Disposal
Isopropyl ether is burned in a chemicalincinerator equipped with an afterburner andscrubber. A small amount of ether, if free ofperoxides, can be evaporated in a fume hoodin the absence of any open flame or sourceof ignition nearby.
Diisopropyl ether Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| TopScience Biochemical | 00852-68527855 | info@itopbiochem.com | China Hong Kong | 902 | 58 |
View Lastest Price from Diisopropyl ether manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-26 | Diisopropyl ether
108-20-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-03-06 | Isopropyl ether
108-20-3
|
US $10.70 / Kg/Drum | 10g | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2022-11-24 | Diisopropyl Ether
108-20-3
|
US $0.00 / ton | 5ton | 99% | 1000tons | Shandong Promise New Material Co.,Ltd. |
-

- Diisopropyl ether
108-20-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Isopropyl ether
108-20-3
- US $10.70 / Kg/Drum
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Diisopropyl Ether
108-20-3
- US $0.00 / ton
- 99%
- Shandong Promise New Material Co.,Ltd.