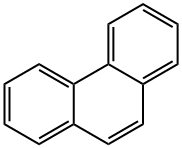
Phenanthrene
- CAS No.
- 85-01-8
- Chemical Name:
- Phenanthrene
- Synonyms
- Phenanthren;31055;Philippines;phenanthrene, pure;ravatite;Phenantren;Phenathrene;Phenanthrenetech;phethane;Phenantrin
- CBNumber:
- CB8854465
- Molecular Formula:
- C14H10
- Molecular Weight:
- 178.23
- MDL Number:
- MFCD00001168
- MOL File:
- 85-01-8.mol
- MSDS File:
- SDS
| Melting point | 98-100 °C (lit.) |
|---|---|
| Boiling point | 340 °C (lit.) |
| Density | 1.063 g/mL at 25 °C (lit.) |
| vapor density | 6.14 |
| vapor pressure | 0.00012 hPa (20 °C) |
| refractive index | 1.5943 |
| Flash point | 99-101°C |
| storage temp. | room temp |
| solubility | Soluble in alcohol, benzene, toluene, and glacial acetic acid |
| pka | >15 (Christensen et al., 1975) |
| form | platelets (fine) |
| color | brown |
| Water Solubility | insoluble |
| Merck | 14,7212 |
| BRN | 1905428 |
| Henry's Law Constant | 0.49, 1.80, 3.35, and 7.89 at 5, 15, 25, and 35 °C, respectively (gas stripping-GC, Odabasi et al., 2006) |
| Exposure limits | OSHA: TWA 0.2 mg/m3 |
| Dielectric constant | 2.72(43.0℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 85-01-8(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 448J8E5BST |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| NIST Chemistry Reference | Phenanthrene(85-01-8) |
| EPA Substance Registry System | Phenanthrene (85-01-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H410 | |||||||||
| Precautionary statements | P264-P270-P273-P301+P312-P391-P501 | |||||||||
| Hazard Codes | Xn,N,F,T | |||||||||
| Risk Statements | 22-36/37/38-50-50/53-40-67-65-38-11-52/53-39/23/24/25-23/24/25-63-43-45-20/21/22-20 | |||||||||
| Safety Statements | 26-60-61-36/37-29-62-45-16-7-24/25-23-53-37/39-22-33-25-9 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SF7175000 | |||||||||
| F | 8 | |||||||||
| Autoignition Temperature | >450 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2902 90 00 | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 i.p. in mice: 700 mg/kg (IARC) | |||||||||
| NFPA 704 |
|
Phenanthrene price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22122 | Phenanthrene for synthesis | 85-01-8 | 100G | $177 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22122 | Phenanthrene for synthesis | 85-01-8 | 250G | $353 | 2024-03-01 | Buy |
| Sigma-Aldrich | 695114 | Phenanthrene sublimed grade, ≥99.5% | 85-01-8 | 1g | $46.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 48569 | Phenanthrene analytical standard, for environmental analysis | 85-01-8 | 5000mg | $125 | 2024-03-01 | Buy |
| Sigma-Aldrich | 40079 | Phenanthrene solution certified reference material, 5000?μg/mL in methanol | 85-01-8 | 1mL | $123 | 2024-03-01 | Buy |
Phenanthrene Chemical Properties,Uses,Production
Non-linear polycyclic aromatic hydrocarbons
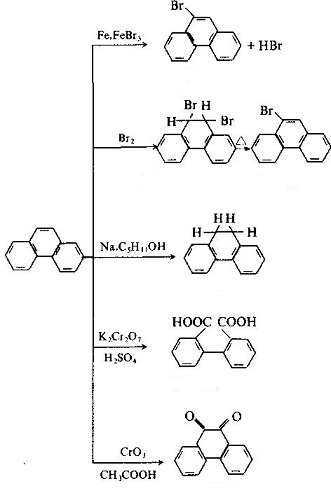
Phenanthrene is the simplest non-linear polycyclic aromatic hydrocarbons with three benzene ring structure, being the isomer of the anthracene. In 1872 E. Ostermayer et al has identified the phenanthrene in the anthracene oil fraction in coal tar distillate, being one of coal tar processing products. In the high-temperature coal tar, the phenanthrene content is secondary only to naphthalene, being about 4~6%, mainly concentrated in the anthracene oil fractions. The chemical activity of phenanthrene is stronger than that of naphthalene, but it is weaker than that of anthracene, and the oxidation and addition reactions can also occur at 9 and 10 positions.
The phenanthrene is a colorless crystal with luster, and the phenanthrene precipitated from ethanol is a colorless monoclinic crystal. The phenanthrene is a leaf-like crystal with a relative density of 1.179 (25/4 ℃) and a refractive index of 1.6450, melting point of 101 °C and boiling point of 340 °C. It can subject to sublimation, being insoluble in water, slightly soluble in ethanol, soluble in ether, benzene, acetic acid, chloroform, carbon tetrachloride and carbon disulfide. The solution exhibits blue fluorescence. The 1, 4, 5, 8-positions are the same, known as α-position; the 2, 3, 6, 7-position are also the same, known as β-position; the 9, 10-positions are the same, known as the ?-position. Its chemical property is between naphthalene and anthracene. It can also have addition reaction in the 9, 10-position, but not as easy as anthracene. Oxidation also occurs at the 9, 10-position with oxidization giving phenanthrenequinone. Substitution reactions may also occur. It can also be obtained through separation from the anthracene oil fraction of coal tar oil. Phenanthrene can be used in the manufacture of pesticides and dyes, but also be used as the stabilizer of the high efficiency & low toxicity pesticides and smokeless powder explosives.
Phenanthrene can be used to produce dyes, drugs and resins after conversion processing. The oxidation products phenanthrenequinone can be used as dyes, fungicides and polymerization inhibitors; 9, 10-biphenyl dicarboxylic acid is used to manufacture polyester and alkyd resin; 9, 10-dihydro-9-phenathroic acid is a plant growth-stimulating hormone; Perhydrophenanthrene made through hydrogenation of phenanthrene can be used in the production of jet fuel; its sulfonated product, phenanthrene sulfonic acid can be used as binder and tanning.
The phenanthrene-containing mother liquor during the production of refined anthracene using solvent method, after recovery of solvent and further crystallization filtration, can give crude phenanthrene containing 40% phenanthrene.
The crude phenanthrene, after removing of residue solvents in the melting kettle and then rectified in the rectifying tower with 20 theoretical plates, the fractions of 335 to 340 °C are cut out, followed by cooling, crystallization and filtering to obtain the industrial phenanthrene with the phenanthrene content of more than 70%. .
The above information is compiled by Tongtong from Chemicalbook.
Molecular Structure
The molecular structure of phenanthrene and anthracene are similar with each other with all the atoms located in the same plane, but not in the same line, being a closed conjugate system with aromatic property. The 1, 2, 3, 4, 10 positions and 5, 6, 7, 8, 9 positions inside the molecules correspond to each other, respectively, but there were differences in activity at the 5 positions, among which 9 and 10 had higher activity with substitution, oxidation and addition occurring in 9 and 10 positions:
Phenanthrenequinone is a pesticide used as germicide seed dressing, being able to prevent wheat scab, hard smut and sweet potato black spot.
Industrial Phenanthrene is derived from distillation of anthracene oil derived from coal tar distillate. Many kinds of natural products (such as sterols) contain this ring system. Phenanthrene is mainly used in the manufacture of dyes, drugs, high efficiency and low toxicity of pesticides, and can be used as scintillants, smokeless powder stabilizer. Many of the phenanthrene derivatives have carcinogenic physiological effects. Such as:
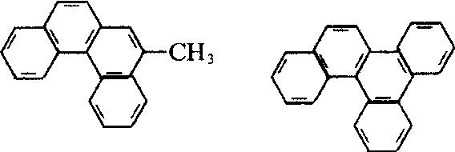
The molecular structure of 2-methyl-3, 4-benzophenanthrene and 1, 2, 3, 4-dibenzophene
Chemical properties
It appears as white luster and fluorescent flake crystals. It is not soluble in water, slightly soluble in ethanol, soluble in ether, acetic acid, benzene, carbon tetrachloride and carbon disulfide.
Uses
It can be used for the manufacturing of phenanthrenequinone, synthetic resin, pesticides and preservatives and so on.
Phenanthrene, through the oxidation, can give phenanthrenequinone, to be used to replace the organic mercurial pesticides ceresin and gallotox. The biphenyl acid obtained from its oxidation can be used to prepare alkyd resin. Phenanthrene oxidation can also give anhydride, cyclohexanone and phenol. The chlorination products of phenanthrene can be used to make non-flammable electrical insulators and impregnants. The sulfonated phenanthrene sulfonic acid can be made of binder, tanning and so on. But in fact most of these applications have yet to be developed. In the paper industry, the Phenanthrene can be used as pulp antifogging agent; can also be used for nitroglycerine explosives and nitrocellulose stabilizer and for the manufacture of smoke bomb; the solid oxide of phenanthrene can be made of excellent flame resistant electrical insulating materials and fillers. In medicine, phenanthrene can be used for synthesizing alkaloids-morphine and caffeine, dimethyl morphine as well as drugs with special physiological effects on many reproductive organs. In the dye industry, the Phenanthrene can be made of 2-aminophenanthrene quinone, benzanthrone, sulfide reduction dye (blue BO, black BB and brown) and so on. In addition, the plastic industry, synthetic tanning agents and phenanthrene, under high temperature and high pressure, can undergo hydrogenation to get hydrophenanthrene, being the fuel of senior jet aircraft.
For the determination of molecular weight and the synthesis of organic compounds.
Preparation
Phenanthrene is a relatively high content of coal tar, accounting for 5% of coal tar, second only to naphthalene content. The anthracene oil in the 300-360 ℃ fraction range of Coal tar has the highest content of Phenanthrene, followed by anthracene and carbazole and so on. The phenanthrene extraction method is usually send anthracene oil for cooling, crystallization, and then vacuum filtration or centrifugal separation for oil separation. The relatively high amount of soluble phenols in oils can be recovered using precision distillation method. The obtained crystal is called crude anthracene, which contains 25-30% anthracene, 22-25% carbazole and 30% phenanthrene. Crude anthracene can be subject to heavy benzene extraction, cooling, filtration with the filtrate steamed out of solvent before recrystallization and filtration. Take filtrate for distillation so we can get industrial phenanthrene with sulfonation to get fine phenanthrene.
Description
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) composed of three fused benzene rings. The name phenanthrene is a composite of phenyl and anthracene. In its pure form, it is found in cigarette smoke and is a known irritant, photosensitizing skin to light. Phenanthrene appears as a white powder having blue fluorescence.
Chemical Properties
Phenanthrene is a white crystalline substance. Weak aromatic odor. Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons.
Physical properties
Colorless, monoclinic crystals with a faint, aromatic odor
Uses
Labelled polycyclic aromatic hydrocarbons as micropollutants.
Uses
Phenanthrene is a PAH that can be derived from coal tar. Phenanthrene is used in the production of dyes, pharmaceuticals, and explosives, and in biochemical research. A derivative, cyclopentanophenanthrene, has been used as a starting material for synthesizing bile acids, cholesterol, and other steroids.
Uses
Phenanthrene is a polycyclic aromatic hydrocarbons, an environmental pollutant.
Production Methods
Phenanthrene occurs in coal tar and can be isolated from several types of crude petroleum.
Definition
ChEBI: A polycyclic aromatic hydrocarbon composed of three fused benzene rings which takes its name from the two terms 'phenyl' and 'anthracene.'
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 30, p. 291, 1993 DOI: 10.1002/jhet.5570300151
The Journal of Organic Chemistry, 18, p. 801, 1953 DOI: 10.1021/jo50013a004
Tetrahedron Letters, 15, p. 495, 1974
General Description
Colorless monoclinic crystals with a faint aromatic odor. Solutions exhibit a blue fluorescence.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenanthrene may react with oxidizing materials .
Health Hazard
The acute oral toxicity of phenanthrene is low.It is more toxic than anthracene. An oral LD50value in mice is reported at 700 mg/kg. It maycause tumor in skin at the site of application.The evidence of carcinogenicity in animals,however, is inadequate.
Fire Hazard
Phenanthrene is combustible.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. Mutation data reported. A human skin photosensitizer. Questionable carcinogen with experimental neoplastigenic and tumorigenic data by skin contact. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes
Potential Exposure
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Carcinogenicity
Phenanthrene is ineffective as an initiator. It is not classifiable as to human carcinogenicity— class 3 by IARC and class D by IRIS, based on no human data and inadequate data from a single gavage study in rats and skin painting and injection studies in mice.
Source
Detected in groundwater beneath a former coal gasification plant in Seattle, WA at a
concentration of 130 μg/L (ASTR, 1995). Detected in 8 diesel fuels at concentrations ranging
from 0.17– 110 mg/L with a mean value of 41.43 mg/L (Westerholm and Li, 1994) and in distilled
water-soluble fractions of new and used motor oil at concentrations of 1.9–2.1 and 2.1–2.2 μg/L,
respectively (Chen et al., 1994). Lee et al. (1992) reported concentration ranges of 100 to 300
mg/L and 15 to 25 μg/L in diesel fuel and the corresponding aqueous phase (distilled water),
respectively. Schauer et al. (1999) reported phenanthrene in diesel fuel at a concentration of 57
μg/g and in a diesel-powered medium-duty truck exhaust at an emission rate of 93.1 μg/km.
Identified in Kuwait and South Louisiana crude oils at concentrations of 26 and 70 ppm,
respectively (Pancirov and Brown, 1975). Diesel fuel obtained from a service station in Schlieren,
Switzerland contained phenanthrene at an estimated concentration of 327 mg/L (Schluep et al.,
2001).
Phenanthrene was detected in asphalt fumes at an average concentration of 57.73 ng/m3 (Wang
et al., 2001).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
625. Phenanthrene was only detected in the water-soluble fraction of diesel fuel at an average
concentration of 17 μg/L.
Based on laboratory analysis of 7 coal tar samples, phenanthrene concentrations ranged from
3,100 to 35,000 ppm (EPRI, 1990). Detected in 1-yr aged coal tar film and bulk coal tar at an
identical concentration of 10,000 mg/kg (Nelson et al., 1996). A high-temperature coal tar
contained phenanthrene at an average concentration of 2.66 wt % (McNeil, 1983). Also identified
in high-temperature coal tar pitches at concentrations ranging from 7,500 to 40,300 mg/kg
(Arrendale and Rogers, 1981). Lee et al. (1992a) equilibrated eight coal tars with distilled water at
25 °C. The maximum concentration of phenanthrene observed in the aqueous phase is 0.4 mg/L.
Nine commercially available creosote samples contained phenanthrene at concentrations
ranging from 48,000 to 120,000 mg/kg (Kohler et al., 2000).
Typical concentration of phenanthrene in a heavy pyrolysis oil is 2.5 wt % (Chevron Phillips,
May 2003).
Environmental Fate
Biological. Catechol is the central metabolite in the bacterial degradation of phenanthrene.
Intermediate by-products include 1-hydroxy-2-naphthoic acid, 1,2-dihydroxynaphthalene, and
salicylic acid (Chapman, 1972; Hou, 1982). It was reported that Beijerinckia, under aerobic
conditions, degraded phenanthrene to cis-3,4-dihydroxy-3,4-dihydrophenanthracene (Kobayashi
and Rittman, 1982).
Soil. The reported half-lives for phenanthrene in a Kidman sandy loam and McLaurin sandy
loam are 16 and 35 d, respectively (Park et al., 1990). Manilal and Alexander (1991) reported a
half-life of 11 d in a Kendaia soil.
Surface Water. In a 5-m deep surface water body, the calculated half-lives for direct photochemical
transformation at 40 °N latitude in the midsummer during midday were 59 and 69 d with
and without sediment-water partitioning, respectively (Zepp and Schlotzhauer, 1979).
Photolytic. A carbon dioxide yield of 24.2% was achieved when phenanthrene adsorbed on
silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985). In a 2-wk
experiment, [14C]phenanthrene applied to soil-water suspensions under aerobic and anaerobic
conditions gave 14CO2 yields of 7.2 and 6.3%, respectively (Scheunert et al., 1987). Matsuzawa et
al. (2001) investigated the photochemical degradation of five polycyclic aromatic hydrocarbons in
diesel particulate matter deposited on the ground and in various soil components. The
photochemical degradation by artificial sunlight was accomplished using a 900-W xenon lamp.
Light from this lamp was passed through a glass filter to eliminate light of shorter wavelengths (λ
<290 nm). The intensity of this light was about 170 mW/cm2. In addition, a solar simulator
equipped with a 300-W xenon lamp was used to provide the maximum sunlight intensity observed
in Tokyo (latitude 35.5 °N). The half-lives of phenanthrene in diesel particulate matter using 900-
and 300-W sources were 4.29 and 60.63 h, respectively. The following half-lives were determined
for phenanthrene adsorbed on various soil components using 900-W apparatus: 3.04 h for quartz,
2.90 h for feldspar, 1.15 h for kaolinite, 4.97 h for montmorillonite, 3.26 h for silica gel, and 1.17
h for alumina.
Chemical/Physical. The aqueous chlorination of phenanthrene at pH <4 produced phenanthrene-
9,10-dione and 9-chlorophenanthrene. At high pH (>8.8), phenanthrene-9,10-oxide, phenanthrene-
9,10-dione, and 9,10-dihydrophenanthrenediol were identified as major products (Oyler et al.,
1983). It was suggested that the chlorination of phenanthrene in tap water accounted for the
presence of chloro- and dichlorophenanthrenes (Shiraishi et al., 1985).
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Likely contaminants include anthracene, carbazole, fluorene and other polycyclic hydrocarbons. Purify it by distillation from sodium under vacuum, boiling with maleic anhydride in xylene, crystallisation from acetic acid, sublimation and zone melting. It has also been recrystallised repeatedly from EtOH, *benzene or pet ether (b 60-70o), with subsequent drying under vacuum over P2O5 in an Abderhalden pistol. Feldman, Pantages and Orchin [J Am Chem Soc 73 4341 1951] separated most of the anthracene impurity by refluxing phenanthrene (671g) with maleic anhydride (194g) in xylene (1.25L) under nitrogen for 22hours, then filtered. The filtrate was extracted with aqueous 10% NaOH, the organic phase was separated, and the solvent was evaporated. The residue, after stirring for 2hours with 7g of sodium, was distilled in a vacuum, then recrystallised twice from 30% *benzene in EtOH. It was then dissolved in hot acetic acid (2.2mL/g), and to it was slowly added an aqueous solution of CrO3 (60g in 72mL H2O plus 2.2L of acetic acid), followed by slow addition of conc H2SO4 (30mL). The mixture was refluxed for 15minutes, diluted with an equal volume of water and cooled. The precipitate was filtered off, washed with water, dried and distilled, then recrystallised twice from EtOH. Further purification is possible by chromatography from a CHCl3 solution on activated alumina, with *benzene as eluent, and by zone refining. The picrate (1:1) forms golden yellow needles with m 146o, and the styphnate (1:1) has m 138-139o (plates or needles from EtOH or EtOH/H2O respectively). [Dornfeld et al. Org Synth Coll Vol III 134 1955, Beilstein 5 H 667, 5 I 327, 5 II 579, 5 III 2136, 5 IV 2297.]
Toxicity evaluation
Phenanthrene absorbs ultraviolet light and causes production of singlet oxygen, which in turn leads to free radical production. Although a large body of literature exists on the toxicity and carcinogenicity of other PAHs, primarily benzo(a)pyrene, toxicity data for phenanthrene are limited.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Phenanthrene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2966 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from Phenanthrene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-19 | Phenanthrene
85-01-8
|
US $0.00 / Kg/Drum | 1KG | 99% | 100mt/month | Jinan Finer Chemical Co., Ltd | |
 |
2023-09-07 | Phenanthrene
85-01-8
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-03-06 | Phenanthrene
85-01-8
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD |
-

- Phenanthrene
85-01-8
- US $0.00 / Kg/Drum
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Phenanthrene
85-01-8
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Phenanthrene
85-01-8
- US $5.00-2.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD