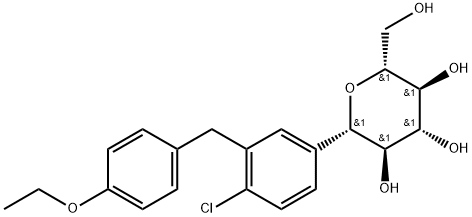
Dapagliflozin
- CAS No.
- 461432-26-8
- Chemical Name:
- Dapagliflozin
- Synonyms
- Dapagliflozin Propanediol Monohydrate;Dapagliflozin propanediol;Farxiga;DAPAGLIFLOZIN BASE;Dapagliflozi;(1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol;D-Glucitol, 1,5-anhydro-1-C-(4-chloro-3-((4-ethoxyphenyl)methyl)phenyl)-, (1S)-;2-(3-(4-Ethoxybenzyl)-4-chlorophenyl)-6-hydroxymethyltetrahydro-2H-pyran-3,4,5-triol;(2S,4R,5R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxyMethyl)tetrahydro-2H-pyran-3,4,5-triol;CS-179
- CBNumber:
- CB91011730
- Molecular Formula:
- C21H25ClO6
- Molecular Weight:
- 408.88
- MDL Number:
- MFCD13182359
- MOL File:
- 461432-26-8.mol
| Melting point | 55-58°C |
|---|---|
| Boiling point | 609.0±55.0 °C(Predicted) |
| Density | 1.349 |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 13.23±0.70(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| Stability | Hygroscopic |
| FDA UNII | 1ULL0QJ8UC |
| NCI Drug Dictionary | dapagliflozin |
| ATC code | A10BK01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H372-H318 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P330-P501-P260-P264-P270-P314-P501-P280-P305+P351+P338-P310 | |||||||||
| NFPA 704 |
|
Dapagliflozin price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 11574 | Dapagliflozin ≥98% | 461432-26-8 | 1mg | $33 | 2024-03-01 | Buy |
| Cayman Chemical | 11574 | Dapagliflozin ≥98% | 461432-26-8 | 5mg | $90 | 2024-03-01 | Buy |
| Cayman Chemical | 11574 | Dapagliflozin ≥98% | 461432-26-8 | 10mg | $164 | 2024-03-01 | Buy |
| Cayman Chemical | 11574 | Dapagliflozin ≥98% | 461432-26-8 | 50mg | $367 | 2024-03-01 | Buy |
| TRC | D185370 | Dapagliflozin | 461432-26-8 | 10mg | $120 | 2021-12-16 | Buy |
Dapagliflozin Chemical Properties,Uses,Production
Diabetes drugs
Dapagliflozin (ForxigaTM) is a new antidiabetic drug jointly developed by Bristol-Myers Squibb and AstraZeneca, being approved by the European Medicines Agency (EMA) on November 12, 2012. It is also the first approved SGLT2 inhibitor for the treatment of type II diabetes, being an important option in the treatment of diabetes, and is used to improve glycemic control as an adjunct to dietary and exercise for adults with type II diabetes.
Dapagliflozin is a sodium-glucose co-transporter 2 inhibitor. On January 8, 2014, the US Food and Drug Administration (FDA) have approved it for being used in the treatment of type II diabetes. Meanwhile, FDA requires the producers to conduct post-marketing research on drug-related risks.
The post-marketing trial requested by the FDA includes a cardiovascular outcome trial for assessing the cardiovascular risk for high-risk patients after treatment with dapagliflozin at baseline and a study to assess the risk of bladder cancer in recruited patients. Another study will assess the bladder tumor-promoting effect of this drug on rodent animals. Two studies will assess the pharmacokinetics, efficacy and safety of dapagliflozin in pediatric patients; a set of strengthened pharmacovigilance program will monitor liver abnormalities and pregnancy outcome reports in patients receiving daglitazone. Dapagliflozin will be marketed under the tradename Farxiga by Haoeyou Pharmacy.
The above information is edited by Andy of chemicalbook.
Pharmacological effects
Dapagliflozin works through inhibiting sodium-glucose transporter 2 (SGLT2), a protein in the kidney that reabsorbs glucose into the bloodstream. This allows extra glucose to be excreted through the urine, improving glycemic control without increasing insulin secretion. The use of this drug requires patients with normal renal function while patients of moderate to severe renal insufficiency should be disabled to use this drug. Single application of this product or combination with metformin, pioglitazone, glimepiride, insulin and other drugs can significantly reduce the HbA1c and fasting blood glucose of patients suffering type II diabetes. The frequency of the adverse reaction was similar to placebo with low risk of hypoglycemia, being able to reduce body weight.
The efficacy of dapagliflozin is comparable with the dipeptidyl peptidase inhibitors, and several new hypoglycemic drugs, and can also mildly lower the blood pressure and body weight. The drug has 5mg and 10mg two tablets to choose from, can be either used alone or together with insulin, including other diabetes drugs.
Pharmacokinetics
In healthy subjects, dapagliflozin was rapidly absorbed after oral administration with a peak time Tmax being 1 to 2 hours, a protein binding rate of 91%, an oral bioavailability of about 78% and a plasma terminal half-life of 12.9 hours. After oral administration, the drug is mainly metabolized by the uridine diphosphate glucuronosyltransferase 1A9 (UGT1A9) into the inactive metabolite in the liver with the smaller part being metabolized by the P450 enzyme and of no inhibitory or inducing effect on the P450 enzyme. Drug prototypes and related metabolites were excreted through urine (75%) and faeces (21%). Compare simultaneous administration of this product with high-fat food and with the fasting administration, Tmax can be extended by 1-fold, but the absorption did not affect the degree, so can be administrated together with the food.
The pharmacokinetics of daglitazone was significantly affected by renal function. Diabetic patients with mild, moderate or severe renal insufficiency are merged to be subject to oral administration of 20 mg • d-1 daglitazone for 7 days. The mean systemic exposure amount, compared with patients with normal renal function, is respectively 32%, 60% and 87% higher. For patients with normal renal function, mild insufficiency, moderate insufficiency and severe insufficiency, the urinary glucose excretion amount in 24 hours of steady state was 85, 52, 18 and 11g, successively.
Kasichayanula et al have studied the pharmacokinetic effects of liver dysfunction on daglitazone. The patients with mild, moderate and severe hepatic insufficiency having a single oral dose of 10 mg of daglitazone, the Cmax of each group was 12% lower, 12% higher and 40% higher than that with normal liver function, respectively. The AUC of each group was significantly higher than that of normal liver function by 3%, 36% and 67%.
Therefore, it is not recommended to apply daglitazone to patients of moderate and severe renal dysfunction. Severe liver dysfunction patients need to reduce the use of dose.
Synthesis method
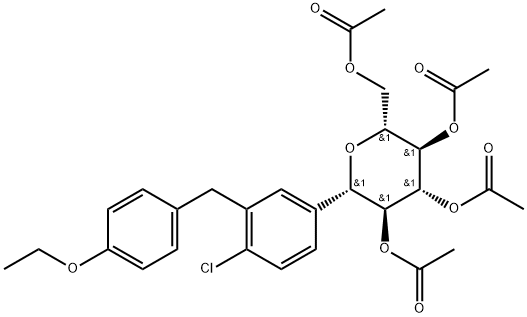
5-bromo-2-chlorobenzoic acid is subject to acylating chlorination, and has Friedel-Crafts reaction with phenylethyl ether for reduction of its carbonyl group, generating 5-bromo-2-chloro-4'-ethoxydiphenyl methane, further subjecting to condensation with 2, 3, 4, 6-tetra-O-trimethylsilyl-D-glucopyranosanoic acid-1,5-lactone. The anomeric carbon hydroxyl group is subject to etherification and deprotection to give 2-chloro-5-(1-methoxy-D-glucopyranose-I-yl)-4'-ethoxydiphenylmethane, and then use Et3SiH/BF3 • OEt2 for reduction to remove methoxy, followed by acetic anhydride esterification and hydrolysis to give hypoglycemic agents daglitazone with the overall yield of about 40%.
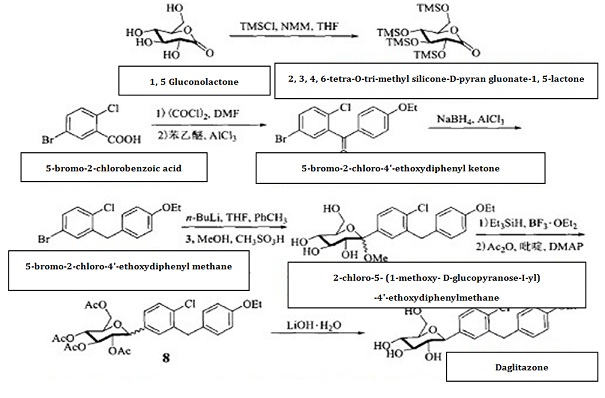
Fig.1 shows the chemical reaction route of synthesizing dapagliflozin.
Safety
Daglitazone has excellent tolerance and safety with the incidence of adverse events associated with 10 mg • d-1 daglitazone being similar to that of placebo. Common adverse events included hypoglycemia, polyuria, back pain, genital infections, urinary tract infections, dyslipidemia and hematocrit (HCT) increase. The overall risk of hypoglycemia is low, and the incidence of hypoglycemia is associated with other basic hypoglycemic agents. The incidence of hypoglycemia was higher in patients subjecting to joint treatment between daglitazone and sulfonylureas or insulin compared with placebo. Therefore, when this product is used in combination with insulin or insulin secretagogue, you may need to adjust the dose of the latter one.
Drug interactions
This product is mainly metabolized in the liver by UGT1A9 metabolism, being the P-glycoprotein substrate. Study confirmed that the pharmacokinetics of daglitazone was not affected by metformin, pioglitazone, sitagliptin, glimepiride, voglibose, and simvastatin, valsartan, warfarin, and digoxin. The serum concentrations of the above-mentioned drugs are also not clinically significantly affected by daglitazone. Rifampicin can reduce the exposure amount of daglitazone by 22% while mefenamic acid can increase the body exposure amount by 51%, but have no clinically significant effect on 24 h urine glucose excretion.
Description
Inhibiting renal glucose reabsorbtion through the sodium-
Description
The Australian Therapeutic Goods Administration (TGA) and the European Commission approved dapagliflozin in October and November 2012, respectively, as an adjunct to diet and exercise for the treatment of type 2 diabetes. Dapagliflozin is a potentially attractive therapy due to its glucosesensitive and insulin-independent mechanism of action. It is a first-in-class selective SGLT2 inhibitor (IC50=1.1 nM; selectivity vs. SGLT1 >1000) that lowers the renal threshold for reabsorption of glucose, allowing excess glucose to be eliminated via the kidneys. In normal rats, administration of dapagliflozin promotes dose-dependent excretion of up to 1900 mg of glucose over a 24 h period, with amaximal effect at 3 mg/kg. In a ratmodel of diabetes, pretreatment with the pancreatic toxin streptozotocin results in hyperglycemia that is reduced 55% by administration of a single 0.1 mg/kg dose of dapagliflozin compared with vehicle. Aryl O-glucoside SGLT2 inhibitors were early entrants into the clinic, but the aryl C-glucoside linkage found in dapagliflozin confers resistance to glucosidase-mediated metabolism leading to improved clinical utility relative to aryl O-glucosides. The modified carbohydrate–aglycone linkage required concomitant adjustment from an ortho- to a meta-substituted arylglucoside to achieve potent SGLT2 inhibition. Dapagliflozin was synthesized in several steps via reaction of an aryllithium with per-silylated gluconolactone to form the key C-glucoside linkage. An alpha-selective reduction of the resultant anomeric glycoside gave the desired beta-Carylglucoside. The main circulating (inactive) metabolite is the result of 3-O-glucuronidation of the glucosylmoiety. Of the minority metabolites, the main oxidative species result from O-dealkylation of the ethoxy-group and hydroxylation of the biarylmethane moiety.
Chemical Properties
White Solid
Originator
Bristol-Myers Squibb (United States)
Uses
therapeutic for diabetes I or II, and hyperglycemia
Uses
A sodium-glucose transporter 2 inhibitor.
Definition
ChEBI: A C-glycosyl comprising beta-D-glucose in which the anomeric hydroxy group is replaced by a 4-chloro-3-(4-ethoxybenzyl)phenyl group. Used (in the formo f its propanediol monohydrate) to improve glycemic ontrol, along with diet and exercise, in adults with type 2 diabetes.
brand name
Forxiga
Clinical Use
Selective and reversible inhibitor of sodium-glucose
co-transporter 2:
Treatment of type 2 diabetes
in vitro
ec50 values of 1.1 nm for hsglt2 and 1.4 μm for hsglt1 determined for dapagliflozin corresponded to 1200-fold selectivity for sglt2 as compared with phlorizin’s 10-fold selectivity. dapagliflozin inhibitory potencies against rat sglt (rsglt)2 and hsglt2 were comparable, but the selectivity of dapagliflozin for rsglt2 versus rsglt1 decreased to 200-fold [1].
in vivo
in vivo, dapagliflozin acutely induced renal glucose excretion in diabetic and normal rats, improved glucose tolerance in normal rats, as well as reduced hyperglycemia in zucker diabetic fatty rats after single oral doses ranging between 0.1 and 1.0 mg/kg [2].
target
hSGLT2
Metabolism
Dapagliflozin is extensively metabolised, primarily to dapagliflozin 3-O-glucuronide, which is an inactive metabolite. The formation of dapagliflozin 3-O-glucuronide is mediated by UGT1A9, an enzyme present in the liver and kidney, and CYP-mediated metabolism was a minor clearance pathway in humans. About 75% of the dose is excreted in the urine and 21% in the faeces.
References
[1] meng w, ellsworth ba, nirschl aa, mccann pj, patel m, girotra rn, wu g, sher pm, morrison ep, biller sa, zahler r, deshpande pp, pullockaran a, hagan dl, morgan n, taylor jr, obermeier mt, humphreys wg, khanna a, discenza l, robertson jg, wang a, han s, wetterau jr, janovitz eb, flint op, whaley jm, washburn wn. discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (sglt2) inhibitor for the treatment of type 2 diabetes. j med chem. 2008 mar 13;51(5):1145-9.
[2] han s, hagan dl, taylor jr, xin l, meng w, biller sa, wetterau jr, washburn wn, whaley jm. dapagliflozin, a selective sglt2 inhibitor, improves glucose homeostasis in normal and diabetic rats. diabetes. 2008 jun;57(6):1723-9.
[3] bailey cj, iqbal n, t'joen c, list jf. dapagliflozin monotherapy in drug-naïve patients with diabetes: a randomized-controlled trial of low-dose range. diabetes obes metab. 2012 oct;14(10):951-9.
Dapagliflozin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Senova Technology Co. Ltd. | +86-0755-86703119 +8618503098836 | info@senovatech.com | China | 349 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9339 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1100 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7786 | 58 |
Related articles
- A kind of preparation method of dapagliflozin
- This article describes a preparation method for dapagliflozin and an overview of its background
- Mar 11,2022
View Lastest Price from Dapagliflozin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Dapagliflozin
461432-26-8
|
US $225.00-120.00 / Kg/Bag | 1Kg/Bag | 99% min / GMP application / PMDA / DMF | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-04-16 | Dapagliflozin
461432-26-8
|
US $5.00-0.10 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2024-04-13 | Dapagliflozin
461432-26-8
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd |
-

- Dapagliflozin
461432-26-8
- US $225.00-120.00 / Kg/Bag
- 99% min / GMP application / PMDA / DMF
- Sinoway Industrial co., ltd.
-

- Dapagliflozin
461432-26-8
- US $5.00-0.10 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Dapagliflozin
461432-26-8
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd