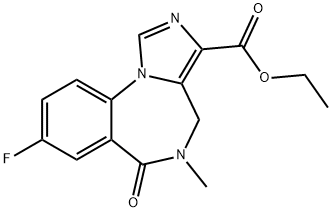
Flumazenil
- CAS No.
- 78755-81-4
- Chemical Name:
- Flumazenil
- Synonyms
- Romazicon;ethyl 8-fluoro-5-Methyl-6-oxo-5,6-dihydro-4H-benzo[f]iMidazo[1,5-a][1,4]diazepine-3-carboxylate;FMZ;YM-684;ANEXATE;Ro 1722;Lanexat;Mazicon;Ro 41-8157;FluMenazil
- CBNumber:
- CB9208108
- Molecular Formula:
- C15H14FN3O3
- Molecular Weight:
- 303.29
- MDL Number:
- MFCD00242764
- MOL File:
- 78755-81-4.mol
- MSDS File:
- SDS
| Melting point | 201-203°C |
|---|---|
| Boiling point | 528.0±50.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Soluble in DMSO to 25mM |
| pka | 0.86±0.20(Predicted) |
| form | solid |
| color | white |
| Water Solubility | 128 mg/L |
| Merck | 14,4135 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 78755-81-4(CAS DataBase Reference) |
| FDA UNII | 40P7XK9392 |
| ATC code | V03AB25 |
| NIST Chemistry Reference | Flumazenil(78755-81-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H303 |
| Precautionary statements | P270-P301+P312-P403-P501c |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-27-36/37/39 |
| WGK Germany | 2 |
| RTECS | NI2922170 |
| HS Code | 2933997500 |
| Toxicity | LD50 in mice, rats (mg/kg): 4000, 1360 i.p.; 4300, 6000 orally (Hunkeler) |
Flumazenil price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | F6300 | Flumazenil >99% (HPLC), solid | 78755-81-4 | 25mg | $402 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2702 | Flumazenil certified reference material, pharmaceutical secondary standard | 78755-81-4 | 500MG | $691 | 2024-03-01 | Buy |
| Sigma-Aldrich | 5.05991 | Flumazenil - CAS 78755-81-4 - Calbiochem | 78755-81-4 | 10mg | $113 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1273808 | Flumazenil United States Pharmacopeia (USP) Reference Standard | 78755-81-4 | 200mg | $2170 | 2024-03-01 | Buy |
| TCI Chemical | F0958 | Flumazenil >99.0%(GC) | 78755-81-4 | 25mg | $91 | 2024-03-01 | Buy |
Flumazenil Chemical Properties,Uses,Production
Description
Flumazenil is a benzodiazepine antagonist useful as a fast-acting antidote in the treatment of benzodiazepine intoxication, and in reversing the central sedative effects of benzodiazepines during anesthesia.
Chemical Properties
Flumazenil is a white to off-white crystalline compound with an octanol:buffer partition coefficient of 14 to 1 at pH 7.4. It is insoluble in water but slightly soluble in acidic aqueous solutions.
Originator
Hoffmann-La Roche (Switzerland)
Uses
Flumazenil is an imidazodiazepine which selectively blocks the central effects of classic benzodiazepines. It is used as benzodiazepine antagonist sedation reversal drug.
Definition
ChEBI: Flumazenil is an organic heterotricyclic compound that is 5,6-dihydro-4H-imidazo[1,5-a][1,4]benzodiazepine which is substituted at positions 3, 5, 6, and 8 by ethoxycarbonyl, methyl, oxo, and fluoro groups, respectively. It is used as an antidote to benzodiazepine overdose. It has a role as a GABA antagonist and an antidote to benzodiazepine poisoning. It is an ethyl ester, an organofluorine compound and an imidazobenzodiazepine.
Preparation
The Synthesis of Flumazenil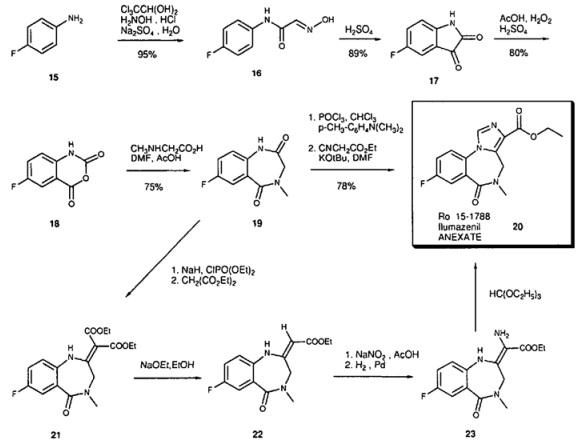
Starting with 4-fluoroaniline (15) the isatin 17 is synthesized via the Sandmeyer synthesis; isatin is then oxidized with peracetic acid to the isatoic anhydride 18. Reaction with sarcosine in DMF leads to the benzodiazepine-2,5-dione 19. This is converted to the iminochloride by reaction with POCI3 . In the key step the imidazoester is built up by reaction with deprotonated ethyl isocyanoacetate [8]. Since ethyl isocyanoacetate is not very stable, an alternative synthesis based on the synthesis of midazolam was developed for large scale-production. Tnthis synthesis diethylmalonate is used. The diester 21 is then transformed to the monoester 22 hy deethoxycarbonylation. Nitrosation and catalytic reduction lead to the amino compound 23. The final carbon atom is introduced by reaction with the orthoester.
brand name
Romazicon (Roche);Anexate.
Therapeutic Function
Benzodiazepine receptor antagonist, Anticonvulsant
Biological Activity
Flumazenil is a GABAA receptor antagonist with non-selective for α 1, α 2, α 3 or α 5 (IC50 = 2 nM in a radioligand binding assay using rat cortical synaptosomes). Flumazenil also acts as a partial agonist of GABAA receptors, decreasing the amplitude of electrically stimulated population spikes in rat hippocampal CA1 pyramidal neurons. It increases the number of entries into the open arms of the elevated plus maze in high-anxiety BALB/c, but not C57BL/6, mice when administered at doses ranging from 0.1 to 1,000 μg/kg. Flumazenil (5 and 10 mg/kg) prevents a reduction in burying behavior induced by the GABAA receptor positive allosteric modulator allopregnanolone in ovariectomized rats when administered at doses of 5 and 10 mg/kg. Formulations containing flumazenil have been used to reverse sedation induced by benzodiazepines and in the treatment of benzodiazepine overdose or withdrawal.
Pharmacokinetics
Flumazenil is a competitive antagonist at the GA BAA benzodiazepine binding site for all other ligands. I t rapidly reverses the CN S and dangerous physiological effects of benzodiazepines following iatrogenic overdose or deliberate self-harm. I t has no effect on benzodiazepine metabolism. Flumazenil is rapidly cleared from plasma and metabolised by the liver and has a very short elimination half-life (<1h). Its duration of action depends on the dose administered and the duration of action of the drug to be antagonised; repeated administration or infusions may be necessary.
Clinical Use
Reversal of sedative effects of benzodiazepines in anaesthetic, intensive care, and diagnostic procedures
Veterinary Drugs and Treatments
Flumazenil may be useful for the reversal of benzodiazepine effects after either therapeutic use or overdoses. Flumazenil may be of benefit in the treatment of encephalopathy in patients with severe hepatic failure.
Metabolism
Flumazenil is extensively metabolised in the liver. The carboxylic acid metabolite is the main metabolite in plasma (free form) and urine (free form and its glucuronide). This main metabolite showed no benzodiazepine agonist or antagonist activity in pharmacological tests.Flumazenil is almost completely (99%) eliminated by non-renal routes. Practically no unchanged flumazenil is excreted in the urine, suggesting complete metabolic degradation of the drug. Elimination of radiolabelled drug is essentially complete within 72 hours, with 90-95% of the radioactivity appearing in urine and 5-10% in the faeces.
storage
+4°C (desiccate)
Mode of action
Flumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on the central nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex. In animal experiments the effects of compounds showing no affinity for the benzodiazepine receptor, e.g. barbiturates, ethanol, meprobamate, GABA mimetics, adenosine receptor agonists and other agents were not affected by flumazenil, but those of nonbenzodiazepine agonists of benzodiazepine receptors, such as cyclopyrrolones (e.g. zopiclone) and triazolopyridazines were blocked.
References
Flumazenil in benzodiazepine overdose
DOI:10.1503/cmaj.160357
Pharmacological uses of flumazenil in benzodiazepine use disorders: a systematic review of limited data
DOI:10.1177/0269881120981390
Flumazenil Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Dorne Chemical Technology co. LTD | +86-13583358881 +86-18560316533 | Ethan@dornechem.com | China | 294 | 58 |
| LY Global chemicals co.,ltd . | +8618939937369 | info@lychems.cn | China | 969 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
| Jinan Million Pharmaceutical Co., Ltd | +86-531-68659554 +8613031714605 | info@millionpharm.com | China | 153 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Shandong Hanjiang Chemical Co., Ltd | +86-0533-2066820 +8618369939125 | hanson@sdhanjiang.com | China | 1527 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 | nova@sh-teruiop.com | China | 525 | 58 |
| Shanghai Aosiris new Material Technology Co., LTD | 86-15139564871 +8615139564871 | wrjmoon2000@163.com | China | 354 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
View Lastest Price from Flumazenil manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-25 | Flumazenil
78755-81-4
|
US $1.00 / g | 1g | 99% | 100kg | Dorne Chemical Technology co. LTD | |
 |
2024-04-25 | Flumazenil
78755-81-4
|
US $1.00-0.50 / kg | 1kg | 99% | 200 | Jinan Million Pharmaceutical Co., Ltd | |
 |
2024-04-17 | Flumazenil
78755-81-4
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd |
-

- Flumazenil
78755-81-4
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Flumazenil
78755-81-4
- US $1.00-0.50 / kg
- 99%
- Jinan Million Pharmaceutical Co., Ltd
-

- Flumazenil
78755-81-4
- US $1.10 / g
- 99.0% min
- Shaanxi Dideu Medichem Co. Ltd