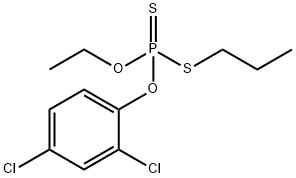
Prothiofos
- CAS No.
- 34643-46-4
- Chemical Name:
- Prothiofos
- Synonyms
- PROTHIOPHOS;TOKUTHION;Bideron;toyodan;Tokution;ntn-8629;toyothion;bayntn8629;PROTHIOFOS;Tokuthhion
- CBNumber:
- CB9385630
- Molecular Formula:
- C11H15Cl2O2PS2
- Molecular Weight:
- 345.25
- MDL Number:
- MFCD00055300
- MOL File:
- 34643-46-4.mol
| Melting point | 25°C |
|---|---|
| Boiling point | 126.5 °C |
| Density | 1.3000 |
| vapor pressure | 3.0×10-4 Pa (20 °C) |
| storage temp. | APPROX 4°C |
| Water Solubility | 0.07 mg l-1(20 °C) |
| form | liquid |
| BRN | 1998314 |
| CAS DataBase Reference | 34643-46-4(CAS DataBase Reference) |
| FDA UNII | H5232196GP |
| NIST Chemistry Reference | Phosphorodithioic acid, o-(2,4-dichlorophenyl) o-ethyl s-propyl ester(34643-46-4) |
| EPA Substance Registry System | Prothiofos (34643-46-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H330-H410 |
| Precautionary statements | P260-P264-P270-P273-P301+P312-P304+P340+P310 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-50/53 |
| Safety Statements | 60-61 |
| RIDADR | UN 3082 |
| WGK Germany | 3 |
| RTECS | TD5680000 |
Prothiofos price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 45311 | Prothiofos PESTANAL | 34643-46-4 | 50mg | $35.2 | 2022-05-15 | Buy |
| TRC | P838840 | Prothiofos | 34643-46-4 | 500mg | $160 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 61420 | Prothiofos | 34643-46-4 | 500mg | $650 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PST0000040 | PROHIFOS 95.00% | 34643-46-4 | 10MG | $885.31 | 2021-12-16 | Buy |
| AK Scientific | S528 | Prothiofos | 34643-46-4 | 50mg | $92 | 2021-12-16 | Buy |
Prothiofos Chemical Properties,Uses,Production
Description
Prothiofos is a colorless liquid. It is nearly insoluble in water (1.7 mg/L at 20 ?C) but readily soluble in most organic solvents. Log Kow = 5.67. It is relatively stable in aqueous media; DT50 values (22 ?C) at pH 4, 7, and 9 are 120, 280, and 12 d, respectively.
Uses
Prothiofos is used to control chewing insects in a range of crops including vegetables, fruit, maize, sugar cane and ornamentals.
Definition
ChEBI: An organic thiophosphate that is the 2,4-dichlorophenyl ester of O-ethyl S-propyl dithiophosphoric acid.
Metabolic pathway
A report by Nihon Tokushu Noyaku Seizo K.K. (1979) (now Nhon Bayer Agrochem K.K.) has summarised the nature of the photolysis products and metabolites formed by prothiofos in plants, insects, chicken, mice, rats, guinea-pigs and rabbits. Major routes for the metabolism of prothiofos include activation via oxidative desulfuration to the oxon and detoxification by dearylation to give 2,4-dichlorophenol which occur in all media. In addition, cleavage of the P-S bond and loss of the propanethiol moiety is an important detoxification mechanism in mammals but not insects and dechlorination by reductive loss of the 2-chlorine substituent in the phenyl ring occurs in soil, plants and photochemically. Stage II metabolism results in the formation of the glucoside of 2,4-dichlorophenol in plants and insects and the glucuronide and sulfate ester in mammals.
Metabolism
The principal metabolic routes are activation by oxidative desulfuraton and detoxification by dearylation and cleavage of the P?S bond in both animals and plants. Prothiofos is strongly adsorbed in soil; the half-life under field conditions is 1–2 months.
Degradation
Prothiofos is hydrolysed at pH 4,7 and 9 with DT50 values of 120,280 and
12 days, respectively (PM). Takase et al. (1982) examined the photolysis
of hexane, methanol, aqueous methanol solutions and thin films of [2H-ethyl]
prothiofos and unlabelled prothiofos. The compound was irradiated
by UV light from a high pressure mercury vapour lamp (λmax 360 nm)
for up to 4 hours or by sunlight for 15 days. Photolysis products were
purified by TLC and identified by GC-MS. Under UV irradiation, prothiofos
was degraded with a half-life of from 60 minutes (hexane solution)
to 420 minutes (thin film). Prothiofos was photolysed under UV light by
five main mechanisms: (a) reductive dechlorination at the 2-position of
the phenyl ring, (b) desulfuration to the oxon ( P=O ) products, (c) cleavage
of the P-S bond and loss of propanethiol, (d) cleavage of the P-O-aryl
linkage resulting in the production of phenols and (e) dechlorination at
the 4-position of the phenyl ring. The main photochemical reaction product
was formed by reductive dechlorination of the 2-position of the
phenyl ring to give 4-chloroprothiofos (2). The next most important
mechanism was photooxidation of the P=S moiety (a common reaction of
phosphorothioates and also noted with parathion, fenitrothion, disulfoton
and fenthoate) to give prothiofos oxon (3), which was subsequently 2-
dechlorinated to give 4. Loss of propanethiol via cleavage of the P-S bond
of prothiofos oxon (3) and the 2-dechlorinated oxon (4) afforded 5 and 6,
respectively, in aqueous solution. In hexane solution photoproducts 7
and 8 were formed by the substitution of the Pr-S group by a chlorine
atom from the ring.
Dearylation by cleavage of the P-O-aryl linkage gave rise to 2,4-
dichlorophenol(9) and 4-chlorophenol(10) which were formed in greater
yields in the aqueous media.4-Dechlorination was a minor route, with only a trace of the di-dechloroprothiofos photoproduct (11) being
detected. In hexane solution only, a number of other photoproducts were
formed of which the most interesting (12) was formed via the displacement
of the 2-chlorine atom by the P-S sulfur (Scheme 1).
Under natural sunlight conditions in hexane the level of photodegradation
was considerably less. The major photoproducts were prothiofos
oxon (3), 4-chloroprothiofos (2) and its oxon (4) and the cyclic
phosphorodithioate (12).
Toxicity evaluation
The acute oral LD50 for rats is 1390–1569 mg/kg. Inhalation LC50 (4 h) for rats is >2.7mg/L air. NOEL (2 yr) for rats is 5mg/kg diet (0.25 mg/kg/d). ADI is 0.1 μg/kg b.w. Prothiofos administered to rats is rapidlymetabolized, and 98% of the dose is excreted in 72 h.
Prothiofos Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | +8613627253706 | sale@hdimpurity.com | China | 1504 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
View Lastest Price from Prothiofos manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-02-02 | Prothiofos
34643-46-4
|
US $1.00 / KG | 1KG | Min98%HPLC | g/kg/ton | Career Henan Chemical Co |
-

- Prothiofos
34643-46-4
- US $1.00 / KG
- Min98%HPLC
- Career Henan Chemical Co