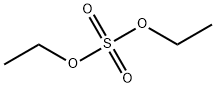
Diethyl sulfate
- CAS No.
- 64-67-5
- Chemical Name:
- Diethyl sulfate
- Synonyms
- DIETHYL SULPHATE;DS;ETHYL SULFATE;SULFURIC ACID DIETHYL ESTER;DHYS;Ethylsulpate;Diethylsulfat;Diaethylsulfat;DIETHYL SULFATE;Two ethyl sulfate
- CBNumber:
- CB9483493
- Molecular Formula:
- C4H10O4S
- Molecular Weight:
- 154.18
- MDL Number:
- MFCD00009099
- MOL File:
- 64-67-5.mol
- MSDS File:
- SDS
| Melting point | -24 °C (lit.) |
|---|---|
| Boiling point | 208 °C (lit.) |
| Density | 1.177 g/mL at 25 °C (lit.) |
| vapor density | 5.3 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 173 °F |
| storage temp. | Refrigerator (+4°C) |
| solubility | soluble in Ether,Alcohol |
| form | Liquid |
| color | Clear |
| Odor | char. faint ether odor, irritating after-effect |
| Water Solubility | 5 g/L (20 ºC) |
| Merck | 14,3130 |
| BRN | 1209714 |
| Dielectric constant | 29.0(20℃) |
| Stability | Moisture sensitive. May react violently with oxidizing agents. |
| CAS DataBase Reference | 64-67-5(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | DIETHYL SULFATE |
| EWG's Food Scores | 4-7 |
| FDA UNII | K0FO4VFA7I |
| Proposition 65 List | Diethyl Sulfate |
| NIST Chemistry Reference | Sulfuric acid, diethyl ester(64-67-5) |
| IARC | 2A (Vol. 54, 71) 1999 |
| EPA Substance Registry System | Diethyl sulfate (64-67-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312+H332-H314-H340-H350 | |||||||||
| Precautionary statements | P202-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 45-46-20/21/22-34 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | UN 1594 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WS7875000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29209090 | |||||||||
| Toxicity | LD50 orally in rats: 0.88 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Diethyl sulfate price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D100706 | Diethyl sulfate 98% | 64-67-5 | 2kg | $99.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | D100706 | Diethyl sulfate 98% | 64-67-5 | 1kg | $106 | 2024-03-01 | Buy |
| TCI Chemical | D0525 | Diethyl Sulfate >98.0%(GC) | 64-67-5 | 25mL | $19 | 2024-03-01 | Buy |
| TCI Chemical | D0525 | Diethyl Sulfate >98.0%(GC) | 64-67-5 | 500mL | $30 | 2024-03-01 | Buy |
| Alfa Aesar | L14291 | Diethyl sulfate, 98% | 64-67-5 | 100ml | $25.65 | 2024-03-01 | Buy |
Diethyl sulfate Chemical Properties,Uses,Production
Description
Diethyl sulfate is a colorless, oily liquid witha faint peppermint-like or ether-like odor. Turns brownon contact with air. Molecular weight=154.20; Boilingpoint=209℃ (decomposes); Freezing/Melting point=225℃; Flash point=104℃; Autoignitiontemperature=436℃. Hazard Identification (based onNFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 1. Insoluble in water; slight decomposition.
Chemical Properties
Diethyl sulfate is a colorless, oily liquid with a faint peppermint- like odor, which darkens with age (Budavari, 1996). It is miscible with alcohol and ether (O'Neil, 2006). At higher temperatures, DES rapidly decomposes into monoethyl sulfate and alcohol (NTP, 2011).
Uses
The primary use of diethyl sulfate is as a chemical intermediate (ethylating agent) in synthesis of ethyl derivatives of phenols, amines, and thiols; as an accelerator in the sulfation of ethylene; and in some sulfonation processes. It is used to manufacture dyes, pigments, carbonless paper, and textiles. It is an intermediate in the indirect hydration (strong acid) process for the preparation of synthetic ethanol from ethylene. Smaller quantities are used in household products, cosmetics, agricultural chemicals, pharmaceuticals, and laboratory reagents (IARC 1992, 1999, HSDB 2009). In 1966, it was used as a mutagen to create the Luther variety of barley (IARC 1974).
Application
Diethyl sulfate can be used as a reactant for the synthesis of:
Biologically active compounds such as bispyrazole, pyrazolopyrimidine and pyridine containing antipyrinyl moieties.
N-substituted-2-styryl-4(3H)-quinazolinones.
Ionic liquids with pyrrolidinium, piperidinium and morpholinium cations, having potential applications as electrolytes.
It can also be used as an alkylating agent to synthesize 1-alkyl/aralkyl-2-(1-arylsufonylalkyl)benzimidazoles and an ionic liquid ethylmethylimidazole ethylsulfate ([EMIM][EtSO4].
Preparation
Diethyl sulfate is produced from ethanol and sulfuric acid, from ethylene and sulfuric acid, or from diethyl ether and fuming sulfuric acid (Budavari, 1996).
Definition
ChEBI: Diethyl sulfate is the diethyl ester of sulfuric acid. It has a role as a carcinogenic agent, an apoptosis inducer, an alkylating agent and a mutagen.
General Description
Diethyl sulfate appears as a clear colorless liquid with a peppermint odor. Burns, though may be difficult to ignite. Corrosive to metals and tissue. It is a potent alkylating agent.
Air & Water Reactions
Highly flammable. Slowly reacts with water to form ethyl alcohol, a flammable liquid, and ethyl sulfate, with eventual conversion to sulfuric acid.
Reactivity Profile
The presence of moisture in a metal container of Diethyl sulfate caused the formation of sulfuric acid which reacts with the metal to release hydrogen which pressurized and exploded the container [Chem. Abst. 28:2908(1934)].
Health Hazard
May be fatal if inhaled, swallowed or absorbed through skin. Inhalation causes nausea and vomiting. Causes burns to skin and eyes. Ingestion may cause nausea, vomiting abdominal pain and collapse.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by inhalation and subcutaneous routes. Moderately toxic by ingestion and sktn contact. A severe skin irritant. An experimental teratogen. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidzing materials. Moisture causes liberation of H2SO4. Violent reaction with potassium tert-butoxide. Reacts violently with 3,8-dnitro-6-phenylphenanthridine + water. Reaction with iron + water forms explosive hydrogen gas. To fight fire, use alcohol foam, H2O foam, CO2, dry chemicals. When heated to decomposition it emits toxic fumes of SOx. See also SULFATES.
Potential Exposure
Tumorigen,Mutagen; Reproductive Effector; Primary Irritant. Used asan industrial chemical; ethylating agent (synthesis of dyeand pharmaceuticals); synthesizing agent of ammoniumchloride compounds; in making isopropyl alcohol, ethylalcohol, and other chemicals; dehydrating agent; extractantfor gasoline.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Gastric lavage (stomach wash), if swallowed,followed by saline catharsis. Do not make an unconsciousperson vomit. Medical observation is recommended for24-48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
Diethyl sulfate is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
storage
(1) Color Code—Yellow Stripe (strong reducingagent): Reactivity Hazard; Store separately in an area isolated from flammables, combustibles, or other yellow-codedmaterials. (2) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Protect against physicaldamage. Store in a cool, dry, well-ventilated location awayfrom any area where the fire hazard may be acute. Outsideor detached storage is preferred. Separate from other storage. Before entering confined space where DS may be present, check to make sure that an explosive concentrationdoes not exist. Store in tightly closed containers in a cool,well-ventilated area. Metal containers involving the transferof this chemical should be grounded and bonded. Wherepossible, automatically pump liquid from drums or otherstorage containers to process containers. Drums must be equipped with self-closing valves, pressure vacuum bungs,and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers ofthis chemical. Sources of ignition, such as smoking andopen flames, are prohibited where this chemical is used,handled, or stored in a manner that could create a potentialfire or explosion hazard. Wherever this chemical is used,handled, manufactured, or stored, use explosion-proof electrical equipment and fittings. A regulated, marked areashould be established where this chemical is handled, used,or stored in compliance with OSHA Standard 1910.1045.
Shipping
This compound requires a shipping label of“POISONOUS/TOXIC MATERIALS.” It falls in HazardClass 6.1 and Packing Group II.
Purification Methods
Wash the ester with aqueous 3% Na2CO3 (to remove acidic material), then distilled water, dry (CaCl2), filter and distil it in a vacuum. It is an ethylating agent and blisters the skin. [Beilstein 1 IV 1236.]
Incompatibilities
Vigorous reaction with strong oxidizersor water (forms sulfuric acid). The aqueous solution is astrong acid; incompatible with nonoxidizing mineral acids,organic acids, bases, acrylates, aldehydes, alcohols, alkylene oxides, ammonia, aliphatic amines, alkanolamines, aromatic amines, amides, chlorates, epichlorohydrin,fulminates, glycols, isocyanates, ketones, powdered metals,organic anhydrides, perchlorates, picrates, substituted allyls,phenols, and cresols. DS decomposes when heated, producing diethyl ether and fumes of sulfur oxides. DS is a strongreducing agent; reacts with oxidizing materials. Formsexplosive hydrogen gas on contact with iron in the presenceof water.
Diethyl sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12453 | 58 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2931 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 47465 | 58 |
View Lastest Price from Diethyl sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-27 | Diethyl sulfate
64-67-5
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2023-06-25 | Diethyl sulfate
64-67-5
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-03-06 | Diethyl sulfate
64-67-5
|
US $10.70 / Kg/Drum | 10g | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD |
-

- Diethyl sulfate
64-67-5
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- Diethyl sulfate
64-67-5
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Diethyl sulfate
64-67-5
- US $10.70 / Kg/Drum
- 99%
- Hebei Guanlang Biotechnology Co,.LTD