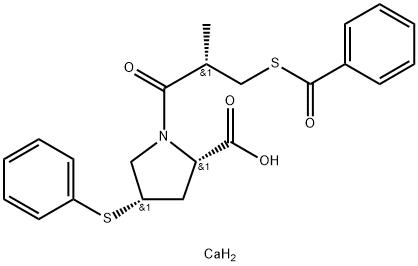
Zofenopril calcium
- CAS No.
- 81938-43-4
- Chemical Name:
- Zofenopril calcium
- Synonyms
- Bifril;Zofenil;SQ26991;Zoprace;Bifril-d5;Zofenil-d5;SQ26991;ZOPRACE;ZOFENOPRIL CALCIUM;ZOFENOPRIL CALCIUM SALT;Zofenopril Calciun Salt
- CBNumber:
- CB9789184
- Molecular Formula:
- C22H25CaNO4S2
- Molecular Weight:
- 471.64
- MDL Number:
- MFCD08704648
- MOL File:
- 81938-43-4.mol
| Melting point | >250°C |
|---|---|
| alpha | D23 -67.6° (c = 1 in methanol/HCl) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO: >5mg/mL |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 81938-43-4(CAS DataBase Reference) |
| FDA UNII | 88ZQ329PU2 |
Zofenopril calcium price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | Z0041 | Zofenopril Calcium | 81938-43-4 | 25MG | $34 | 2024-03-01 | Buy |
| TCI Chemical | Z0041 | Zofenopril Calcium | 81938-43-4 | 100MG | $95 | 2024-03-01 | Buy |
| Cayman Chemical | 29525 | Zofenopril (calcium salt) | 81938-43-4 | 5mg | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 29525 | Zofenopril (calcium salt) | 81938-43-4 | 10mg | $96 | 2024-03-01 | Buy |
| Cayman Chemical | 29525 | Zofenopril (calcium salt) | 81938-43-4 | 25mg | $225 | 2024-03-01 | Buy |
Zofenopril calcium Chemical Properties,Uses,Production
Antihypertensives
Zofenopril calcium is an angiotensin converting enzyme inhibitor (ACEI) class of anti-hypertensive drugs, it is successfully developed by the US Bristol-Myers Squibb Company for the first time. It is introduced to market by the Italian Menarini pharmaceutical ,on January 8, 2001, for the first time it is listed in Italy and other European countries, trade names Bifril, Zofenil, Zopranol, it is useful in the treatment of mild to moderate essential hypertension, as well as for acute myocardial infarction patients within 24 hours, symptomatic or asymptomatic, hemodynamically stable and not receiving thrombolytic therapy. This product can significantly reduce mortality of cardiovascular disease in high-risk patients and incidence of life-threatening cardiovascular events, ,which lays a sound basis for the prevention and treatment of cardiovascular disease, showing a bright future. These drugs are more attractive in treatment of patients with hypertension (they have a higher incidence of coronary heart disease risk than the normal blood pressure population ) .
Angiotensin-converting enzyme inhibitors (ACEI) is an important kind of anti-hypertensive drugs, since 1981, the first orally active angiotensin converting enzyme inhibitor (ACEI) captopril was listed, there has been at least 20 species of ACEI on the market, this year under the calcium channel blocker (CCB), a non-peptide angiotensin ⅱ (AngⅡ) receptor inhibitor leading the general trend of the market , ACEI kinds of drugs are still unique, occupying 1/5 of the world cardiovascular drug market share ,they ease the crisis of hundreds of millions of primary, secondary hypertension patients . In recent years a number of large-scale clinical studies have shown that ACEI has a protective effect on the heart, blood vessels and kidneys , especially for heart protection, clinical manifestations:
1. increase coronary blood flow.
2. reduce the left ventricular hypertrophy.
3. reduce the recurrence rate and mortality of myocardial infarction .
4. Mitigate pathological ventricular remodeling and dysfunction.
5. reduce the incidence and mortality of congestive heart failure.
Furthermore, ACEI also achieves gratifying results in the treatment of diabetic nephropathy .
Pharmacological effects
Zofenopril calcium is a long-acting ACE inhibitor containing a mercapto group on the chemical structure, it is a prodrug, having lipophilic character , in vivo it plays efficacy as free thiol group zofenopril by esterase hydrolysis. Because the role of thiol group, it has stronger antioxidant properties. In addition to having the general ACEI effect of the treatment of hypertension and congestive heart failure , it also combines a unique effect on myocardial infarction.
Zofenopril calcium compared to other ACE inhibitors, it has the following advantages:
1. zofenopril and captopril can protect the myocardium, and resist myocardial ischemia, high concentrations of enalapril, ramiprilat, Fosinoprilat have no myocardial protection.
2. strong lipophilic, high concentrations in the heart. zofenopril calcium is 200 times higher than Enalapril , and 1,500 times higher than ramipril in the heart.
3. it can be significant and long-term in cardiac ACE inhibition. Captopril and fosinopril also have this effect, but they have shorter duration of action.
4. it can improve left ventricular function after ischemia, increase coronary blood flow, reduce creatine kinase (CK) release, but fosinopril does not have this effect.
5. against the heart and other target organ damage caused by free radicals, the effect strength is better than others.
Zofenopril calcium ,in addition to similar anti-hypertension and congestive heart failure like other ACE inhibitors ,can prevent and treat myocardial infarction ,it may become the drug of choice for the treatment and prevention of myocardial infarction with hypertension .
The above information is edited by the Chemicalbook of Tian Ye.
Uses
ACEI (angiotensin-converting enzyme inhibitors) class of antihypertensive drugs
Description
Zofenopril is a prodrug form of the angiotensin-converting enzyme (ACE) inhibitor zofenoprilat. Zofenopril is hydrolyzed by cardiac esterases in vivo to form zofenoprilat. It inhibits ACE with an IC50 value of 0.9 nM in heart tissue homogenates and inhibits cardiac ACE activity in isolated perfused rat hearts. It reduces mean arterial blood pressure in two kidney-one clip renal hypertensive (2K-1C) rats and spontaneously hypertensive rats (SHRs) when administered at doses of 2.2, 6.6, and 22 mg/kg. Unlike the ACE inhibitor ramipril , zofenopril does not affect bronchoalveolar lavage fluid (BALF) levels of bradykinin or prostaglandin E2 (PGE2; ) or increase coughing induced by citric acid in guinea pigs.
Description
Zofenopril calcium was introduced as a second-generation angiotensinconverting enzyme (ACE) inhibitor for the treatment of acute myocardial infarction. Zofenopril, rapidly hydrolyzed by cardiac esterase, is actually a S-benzoyl prodrug of the active component zofenopril-sulfhydryl (zofenoprilat), the latter being the ACE inhibitor responsible for the improvement of postischemic contractile function and for the reduction of cardiac cell death. It was suggested that ACE inhibition alone was not sufficient to explain the cardioprotective effects; the antioxidant properties demonstrated in vitro and in viva could partly explain the strong anti-ischemic effects. In rats with CHF after myocardial infarction, zofenopril attenuated ventricular enlargement and cardiac hypertrophy. It was also shown in isolated globally ischemic rat hearts that the cardioprotective effect of zofenopril was stereoselective. Clinical studies in healthy volunteers comparing zofenopril with enalapril demonstrated that the rate of hydrolysis of the former was faster; at 30 or 60 mg, ACE was completely inhibited in most volunteers until 12h after administration. In patients with anterior acute myocardial infarction, a g-week treatment with zofenopril significantly reduced the incidence of death or severe CHF and also improved the chance of surviving the next year.
Chemical Properties
Off-White to Light Yellow Crystalline Solid
Originator
Bristol-Myers Squibb (US)
Uses
Angiotensin-converting enzyme ACE inhibitor. A prodrug that is de-esterified to the active inhibitor, the sulfhydryl group containing metabolite, Zofenoprilat
Uses
adenosine A2a receptor agonist
Definition
ChEBI: An organic calcium salt that is the hemicalcium salt of zofenopril. A prodrug for zofenoprilat.
Manufacturing Process
9.9 g (0.031 mole) of cis-4-phenylthio-L-proline is suspended in 100 ml of
water (pH 5.6) and the pH is adjusted to 10.2 by the addition of about 20 ml
of 10% sodium bicarbonate to provide a clear solution. The pH is then
adjusted to 9.5 by the addition of about 4.5 ml of concentrated HCl. The
solution is kept at 30°C while 8.1 g (0.033 mole) of (D)-3-(benzoylthio)-2-
methylpropanoic acid chloride in 30 ml of toluene is added simultaneously
with 100 ml of 10% sodium bicarbonate to keep the pH at 9.3. After about
1/4 of the acid chloride is added, a slimy precipitate begins to form which
persists throughout the reaction. After stirring the reaction mixture at pH 9.3
for 2.5 h, it is made strongly acidic by adding 20% HCl in the presence of
ethyl acetate. The aqueous layer is extracted twice with 350 ml portions of
ethyl acetate and the combined organic layers are washed with 300 ml of
saturated brine and dried (MgSO 4 ). The solvent is removed to yield 11.8 g of
foamy solid cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline hydrochloride.
To a solution of this cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline hydrochloride 11.8 g (0.027 mole) in 70 ml of
acetonitrile there is added about 6.0 g of dicyclohexylamine in 25 ml of ether.
A white crystalline precipitate forms immediately. After standing overnight in
the cold room, the solid is filtered and washed with ether to yield (cis)-1-[D-
3-(benzoylthio)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline,
dicyclohexylamine salt (1:1).
he slightly moist (cis)-1-[D-3-(benzoylthio)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline dicyclohexylamine salt is stirred for 2.5 h in a mixture
of 300 ml of ethyl acetate and 200 ml of 10% potassium bisulfate. Two clear
layers form. The aqueous layer is extracted with two 200 ml portions of ethyl
acetate and the combined organic layers are dried (MgSO 4 ). The solvent is
removed to yield 10.1 g of foamy solid (cis)-1-[D-3-(benzoylthio)-2-methyl-1-
oxopropyl]-4-(phenylthio)-L-proline; melting point 42-44°C.
In practice it is usually used as calcium salt (2:1).
brand name
Zoprace (Bristol-Myers Squibb);Zantipres.
Therapeutic Function
Antihypertensive
Zofenopril calcium Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Changzhou Ansciep Chemical Co., Ltd. | +86 519 86305871 | sales@ansciepchem.com | CHINA | 4242 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12431 | 58 |
| WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | +8615377521700 | wangwendy93@gmail.com | China | 868 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9553 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 879 | 58 |
View Lastest Price from Zofenopril calcium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-16 | Zofenopril calcium
81938-43-4
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2021-10-20 | Zofenopril calcium
81938-43-4
|
US $1000.00 / KG | 1KG | 99% | 9000kg/per week | Hebei Lingding Biotechnology Co., Ltd. | |
 |
2021-08-10 | Zofenopril calcium USP/EP/BP
81938-43-4
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited |
-

- Zofenopril calcium
81938-43-4
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- Zofenopril calcium
81938-43-4
- US $1000.00 / KG
- 99%
- Hebei Lingding Biotechnology Co., Ltd.
-

- Zofenopril calcium USP/EP/BP
81938-43-4
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited