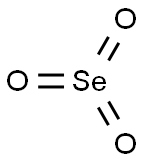Selenium trioxide
- CAS No.
- 13768-86-0
- Chemical Name:
- Selenium trioxide
- Synonyms
- Selenium trioxide;Selenium(VI) oxide;Selenium(VI) trioxide;Selenium trioxide ISO 9001:2015 REACH;Selenium oxide (SeO3) (6CI,7CI,8CI,9CI)
- CBNumber:
- CB9852105
- Molecular Formula:
- O3Se
Lewis structure
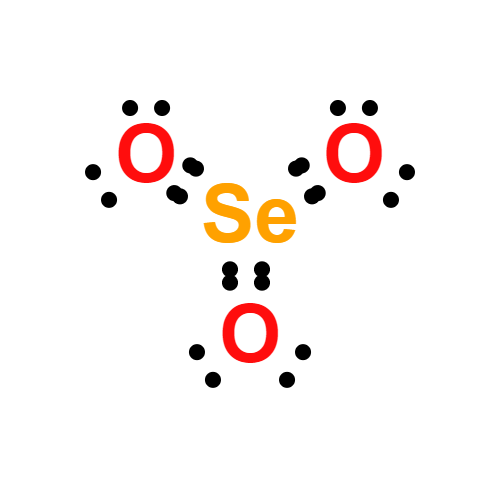
- Molecular Weight:
- 126.96
- MDL Number:
- MOL File:
- 13768-86-0.mol
- MSDS File:
- SDS
| Melting point | 118°C |
|---|---|
| Boiling point | decomposes at 180℃ [CRC10] |
| Density | 3.440 |
| solubility | soluble in H2O, organic solvents |
| form | white tetragonal crystals |
| color | white tetragonal crystals, crystalline; hygroscopic |
| Water Solubility | dissolves in H2O forming selenic acid [KIR82] |
| EWG's Food Scores | 1 |
| EPA Substance Registry System | Selenium oxide (SeO3) (13768-86-0) |
Selenium trioxide Chemical Properties,Uses,Production
Chemical Properties
white crystal(s); hygroscopic; can be prepared by reacting SO3 with potassium selenate or phosphorus pentoxide with selenic acid; strong oxidizing agent; stable in dry air at room temperatures; decomposes, if heated, first to selenium pentoxide, then to the dioxide [KIR82]
General Description
White Solid.
Air & Water Reactions
Reacts vigorously with water to form selenic acid solution.
Reactivity Profile
Inorganic oxidizing agents, such as Selenium trioxide, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds in general have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.
Health Hazard
Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of breath. Inhalation can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness and conjunctivitis. Contact with eyes or skin causes irritation.
Selenium trioxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9604 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9360 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 58 |
| Shaanxi Didu New Materials Co. Ltd | 58 |
| Mainchem Co., Ltd. | 55 |